Mammoth Biosciences secured U.S. DoD award to develop CRISPR-based diagnostics and biosurveillance platforms
On Jan. 14, 2021, Mammoth Biosciences announced that it had secured a subcontract with MRIGlobal, the prime contractor…

On Jan. 14, 2021, Mammoth Biosciences announced that it had secured a subcontract with MRIGlobal, the prime contractor…

On Jan. 14, 2021, Cepheid that Health Canada announced that it had issued Cepheid a medical device license…

On Jan. 13, 2021, researchers announced they had developed a blood test that could make it possible for…

On Jan. 13, 2021, National Institutes of Health scientists and their colleagues announced they had identified a S….

On Jan. 13, 2021, Oxford Immunotec announced the start of a collaboration with Valneva whereby it will perform…

On Jan. 13, 2021, Mateon Therapeutics reported positive interim results from its ARTI-19 clinical trial evaluating ARTIVedaTM against…

On Jan. 13, 2021, the USDAメs (USDA) National Veterinary Services Laboratories announced confirmation of SARS-CoV-2 (the virus that…

On Jan. 13, 2021, Alexion announced the decision to pause further enrollment in the global Phase 3 study…

On Jan. 13, 2021, SIGA Technologies announced that the Public Health Agency of Canada had awarded a contract…

On Jan. 13, 2021, the Oregon Department of Agriculture (ODA) continueed to test, survey, and trap at an…

On Jan. 12, 2021, the four leading international health and humanitarian organizations announced the establishment of a global…

On Jan. 12, 2021, Merck announced the FDA accepted for priority review a Biologics License Application (BLA) for…

On Jan. 12, 2021, Abbott announced it had received FDA 510(k) clearance for the first rapid handheld traumatic…

On Jan. 12, 2021, the WHO announced that global scientists were intensifying research into COVID-19 to expand its…

On Jan. 12, 2021, UC Berkeley HIV researchers announced that they had determined the atomic structure of a…

On Jan. 12, 2021, the Tufts Center for the Study of Drug Development (Tufts CSDD) reported that ever…

On Jan. 12, 2021, Moderna announced that Swissmedic, the Swiss Agency for Therapeutic Products, had authorized the COVID-19…

On Jan. 12, 2021, Regeneron announced that the U.S. Department of Health and Human Services (HHS) and the…
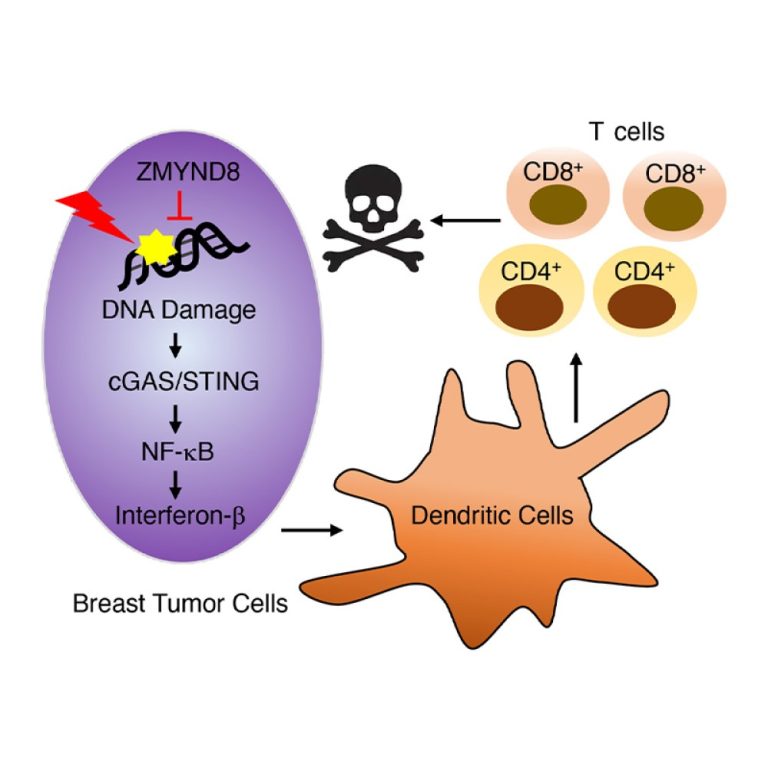
On Jan. 12, 2021, a team researchers from the University of Texas Southwestern (UTSW) announced they had identified…

On Jan. 11, 2021, Roche announced that the European Commission (EC) had approved Xofluza (baloxavir marboxil) for the…

On Jan. 11, 2021, CureVac announced the publication of preclinical data demonstrating the induction of robust antibody and…

On Jan. 11, 2021, Abbott announced it had received FDA 510(k) clearance for the first rapid handheld traumatic…

On Jan. 11, 2021, the FDA conditionally approved Anivive Lifesciences’ Laverdia-CA1 (verdinexor tablets) to treat dogs with lymphoma,…

On Jan. 11, 2021, Editas Medicine announced the U.S. Food and Drug Administration (FDA) had cleared the initiation…

On Jan. 11, 2021, Baxter announced an agreement to provide sterile manufacturing services for NVX-CoV2373, Novavax COVID-19 recombinant…
On Jan. 11, 2021, T2 Biosystems announced that its T2SARS-CoV-2 Panel – a molecular diagnostic test that detects…

On Jan. 11, 2021, Moderna announced that it was expanding its pipeline of innovative vaccines with three new…
On Jan. 11, 2021, AXIM Biotechnologies announced that it had released a preprint of its manuscript describing the…

On Jan. 10, 2021, Humanigen announced that they were partnering to make lenzilumab available to hospitalized and hypoxic…

On Jan. 8, 2021, the Medicines and Healthcare products Regulatory Agency (MHRA) announced it had accepted the recommendation…