FDA approved first extended-release, injectable drug regimen for adults living with HIV
On Jan. 21, 2021, the FDA approved Cabenuva (cabotegravir and rilpivirine, injectable formulation) as a complete regimen for…

On Jan. 21, 2021, the FDA approved Cabenuva (cabotegravir and rilpivirine, injectable formulation) as a complete regimen for…

On Jan. 21, 2021, the U.S. Department of Defense (DOD) recommended that adults ages 75 and older should…
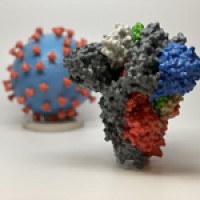
On Jan. 21, 2021, NIH researchers announced that after people recover from infection with a virus, the immune…

On Jan. 21, 2021, scientists at the Wellcome Sanger Institute announced they had created a highly detailed map…

On Jan. 21, 2021, Eli Lilly announced that bamlanivimab (LY-CoV555) significantly reduced the risk of contracting symptomatic COVID-19…

On Jan. 21, 2021, VBI Vaccines announced that it expected to initiate the first Phase 1/2 clinical study…

On Jan. 21, 2021, Moderna announced that the first participant has been dosed in the Phase 1/2 study…

On Jan. 21, 2021, Janssen Pharmaceutical announced the FDA had approved CABENUVA (consisting of Janssenメs rilpivirine and ViiV…

On Jan. 21, 2021, Penn Medicine reported that patients with inactive cancer and not currently undergoing treatments also…

On Jan. 21, 2021, XBiotech announced that its COVID-19 candidate True Humanル antibody therapy may also be used…

On Jan. 19, 2021, a new state of the art institute for antimicrobial research iwasa announced at Oxford…

On Jan. 19, 2021, the Northern Arizona University and TGen, an affiliate of City of Hope, announced results…

On Jan. 19, 2021, ImmunityBio reported findings from a follow-up Molecular Dynamics simulation study of the SARS-CoV-2 spike…

On Jan. 19, 2021, ImmunityBio announced it had received authorization from the South Africa Health Products Regulatory Authority…

On Jan. 19, 2021, Mesa Biotech announced it had received 510(k) clearance and Clinical Laboratory Improvements Amendments (CLIA)…

On Jan. 19, 2021, Cue Health announced that its molecular, point-of-care COVID-19 Tests were being distributed to five…

On Jan. 19, 2021, Heat Biologics announced it had transferred its gp96-based COVID-19 vaccine cell line (“ZVX-60”) to…

On Jan. 19, 2021, Pfizer and BioNTech announced that results from an in vitro study that provided additional…

On Jan. 18, 2021, researchers in Konstanz, Wurzburg, Hamburg and Vienna, led by evolutionary biologist Professor Axel Meyer…

On Jan. 18, 2021, Quest Diagnostics announced that it has entered into an agreement with the Centers for…

On Jan. 15, 2021, scientists studying the bodyメs natural defenses against bacterial infection announced they had identified a…

On Jan. 15, 2021, Oxford University announced that on advice of the independent Data Monitoring Committee (DMC), recruitment…

On Jan. 15, 2021, VBI Vaccines announced that results from a Phase 4 study of VBIメs prophylactic 3-antigen…

On Jan. 15, 2021, scientists at Washington University School of Medicine in St. Louis announced that that a…

On Jan. 15, 2021, Pfizer and BioNTech announced they had developed a plan that allowed the scale-up of…

On Jan. 15, 2021, researchers at the University of Wisconsin School of Medicine and Public Health and UW…

On Jan. 14, 2021, Twist Bioscience announced that it had started shipping its new synthetic RNA reference controls,…

On Jan. 14, 2021, PerkinElmer announced that its PerkinElmerᆴ Coronavirus Nucleic Acid Detection Kit received Emergency Use Authorization…

On Jan. 14, 2021, researchers from the University of Southern California and Princeton reported that the COVID-19 pandemic,…

On Jan. 14, 2021, Mammoth Biosciences announced that it had secured a subcontract with MRIGlobal, the prime contractor…