Study linked structural brain changes to behavioral problems in children who snore
On Apr. 13, 2021, a large study of children has uncovered evidence that behavioral problems in children who…

On Apr. 13, 2021, a large study of children has uncovered evidence that behavioral problems in children who…

On Apr. 13, 2021, Cardinal Health was awarded a $57.8 million contract, including options that if exercised by…

As of Apr. 12, 2021, more than 6.8 million doses of the Johnson & Johnson (Janssen) vaccine were…

On Apr. 13, 2021, the FDA and CDC announced that they were are reviewing data involving six reported…

On Apr. 13, 2021, Rigel Pharma announced the completion of patient enrollment in a multi-center Phase 2 clinical…
On Apr. 13, 2021, Moderna announced that new results from a preclinical study of the Company’s COVID-19 variant-specific…

On Apr. 12, 2021, Resverlogix announced that it had successfully received its desired ‘No Objection Letter’ from Health…
On Apr. 12, 2021, Roche confirmed positive results from the phase III REGN-COV 2069 trial assessing the ability…

On Apr. 12, 2021, Oxford University reported that early treatment with inhaled budesonide shortened recovery time by a…

On Apr. 12, 2021, Thermo Fisher Scientific announced that the U.S. Food and Drug Administration (FDA) had granted…

On Apr. 9, 2021, the American Chemical Society awarded The Priestley Medal to Paul Alivisatos “to recognize distinguished…

On Apr. 9, 2021, Regeneron announced that newly updated National Institutes of Health (NIH) COVID-19 treatment guidelines strongly…
On Apr. 9, 2021, Pfizer announced they had requested amendments to the U.S. Emergency Use Authorization (EUA) of…

On Apr. 8, 2021, Oxford University announced that favipiravir was to be investigated in the UK as part…

On Apr. 8, 2021, ImmunityBio reported initial data indicating that a single subcutaneous injection of the companyメs COVID-19…

On Apr. 8, 2021, Eli Lilly and Incyte announced results of COV-BARRIER, a Phase 3 study evaluating baricitinib…

On Apr. 7, 2021, the National Institutes of Health (NIH) announced that a clinical trial was underway to…
On Apr. 7, 2021, the COVID-19 subcommittee of the WHO Global Advisory Committee on Vaccine Safety (GACVS) announed…

On Apr. 7, 2021, AIM ImmunoTech announced that it had completed dosing of Cohort 1 in a Phase…

On Apr. 7, 2021, Moderna announced publication of antibody persistence data out to 6 months following the second…
On Apr. 7, 2021, McGill University researchers announced that they had developed an innovative tough gel sheathed (TGS)…

On Apr. 7, 2021, the Centers for Disease Control and Prevention (CDC) announced it had awarded $3 billion…

On Apr. 6, 2021, Moderna and Catalent announced the expansion of their strategic collaboration to dedicate a new…

On Apr. 6, 2021, the Centers for Disease Control and Prevention (CDC) reported that nearly 80 percent of…
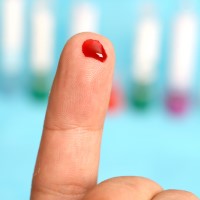
On Apr. 5, 2021, the FDA issued an Emergency Use Authorization (EUA) to Symbiotica for the COVID-19 Self-Collected…

On Apr. 5, 2021, Anixa Biosciences announced that based on Proof of Concept animal study results, it was…

On Apr. 5, 2021, Dynavax Technologies announced that Valneva had reported positive initial results for Part A of…

On Apr. 5, 2021, Innovation Pharmaceuticals announced that an independent Data Monitoring Committee (DMC) completed its scheduled review…

On Apr. 5, 2021, Novavax announced the initiation of crossover arms in two ongoing clinical trials of NVX-CoV2373,…

On Apr. 4, 2021, Emergent BioSolutions announced it had received a contract modification to increase the original task…