Zipline, Pfizer and BioNTech paved way for automated, on-demand delivery of first mRNA COVID-19 vaccines in Ghana
On Nov. 11, 2021, Zipline, Pfizer and BioNTech announced that Zipline had successfully completed the first long-range drone…

On Nov. 11, 2021, Zipline, Pfizer and BioNTech announced that Zipline had successfully completed the first long-range drone…

On Nov. 11, 2021, the University of Oxford began recruiting for a Phase I trial to test an…

On Nov. 10, 2021, a study by researchers at Georgia State University showed that the politicization of science…

On Nov. 10, 2021, the Biden-Harris Administration announced that it was increasing access to COVID-19 testing for Americans…

On Nov. 10, 2021, the World Health Organization (WHO) announced that while reported measles cases had fallen compared…

On Nov. 10, 2021, Meridian Bioscience announced that their Revogeneᆴ SARS-CoV-2 assay was granted Emergency Use Authorization by…

On Nov. 9, 2021, Inovio Pharma announced that the U.S. Food and Drug Administration (FDA) provided authorization to…

On Nov. 9, 2021, Howard Hughes Medical Institute announced a program, named Codetta, that can read the genome…

On Nov. 9, 2021, Swedish announced a $20 million personal bequest from the late philanthropist Paul G. Allen,…

On Nov. 9, 2021, Moderna announced that it has submitted for a variation to the conditional marketing authorization…

On Nov. 8, 2021, Regeneron announced that the European Commission had approved the casirivimab and imdevimab antibody cocktail,…

On Nov. 8, 2021, The U.S. Food and Drug Administration (FDA) reissued the emergency use authorization (EUA) for…

On Nov. 8, 2021, Amyris announced announced it had entered into a 50:50 joint venture arrangement with ImmunityBio…

On Nov. 8, 2021, Cocrystal Pharma announced that its SARS-CoV-2 main protease inhibitors showed potent in vitro pan-viral…

On Nov. 5, 2021, the USDA’s (USDA) National Veterinary Services Laboratories announced confirmation of SARS-CoV-2 in two spotted…

On Nov. 5, 2021, scientists at the National Institutes of Health announced they had found that a process…

On Nov. 5, 2021, the U.S. Food and Drug Administration The FDA issued an emergency use authorization (EUA)…

On Nov. 5, 2021, scientists at Oxford University announced they had identified the gene responsible for doubling the…

On Nov. 5, 2021, Chugai Pharmaceutical, announced that it had obtained approval from the Ministry of Health, Labour…

On Nov. 5, 2021, Pfizer announced it was investigational novel COVID-19 oral antiviral candidate, PAXLOVID, significantly reduced hospitalization…

On Nov. 4, 2021, Novavax announced the completion of its rolling submission to the World Health Organization (WHO)…

On Nov. 3, 2021, the World Health Organization (WHO) issued an emergency use listing (EUL) for COVAXINᆴ (developed…

On Nov. 5, 2021, Resverlogix announced that it had entered into a cooperation agreement with the Supreme Council…

On Nov. 3, 2021, Inovio Pharma announced that it had received authorization from India’s Central Drugs Standard Control…

On Nov. 3, 2021, Novavax announced the company had filed for provisional approval of the vaccine to the…

On Nov. 3, 2021, Innovation Pharma announced that the Company had received confirmation that hard lock of the…
On Nov. 2, 2021, the National Institutes of Health announced support for a four-year follow-up study on the…
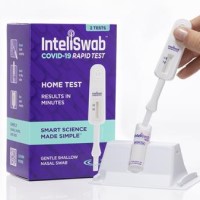
On Nov. 2, 2021, OraSure Technologies announced that the EUA for its InteliSwabル COVID-19 rapid tests had been…

On Nov. 5, 2021, NRx Pharmaceuticals reported a safety update on ZYESAMIᆴ (aviptadil), which was being tested in…

On Nov. 1, 2021, for the first time in the U.S., the transmission of COVID-19 from pet parent…