FDA issued an EUA to the Twist Bioscience for their SARS-CoV-2 NGS assay.
On Mar. 26, 2021, the FDA issued an emergency use authorization (EUA) to the Twist Bioscience for their…

On Mar. 26, 2021, the FDA issued an emergency use authorization (EUA) to the Twist Bioscience for their…

On Mar. 23, 2021, Pfizer announced that it was progressing to multiple ascending doses after completing the dosing…

On Mar. 23, 2021, Omeros and Quantum Leap Healthcare Collaborative announced that dosing of patients with narsoplimab in…

On Mar. 22, 2021, AIM ImmunoTech announced that the Institutional Review Board (IRB) of Roswell Park Comprehensive Cancer…

On Mar. 22, 2021, Oxford University announced that a Phase III study of the Oxford-AstraZeneca coronavirus vaccine conducted…

On Mar. 22, 2021, the NIH announced that results from a large clinical trial in the U.S. and…

On Mar. 22, 2021, Moderna announced that the Philippines had secured 7 million additional doses of COVID-19 Vaccine…

On Mar. 21, 2021, Cepheid announced it had received Emergency Use Authorization from the U.S. Food & Drug…

On Mar. 19, 2021, the FDA issued an emergency use authorization to Tiger Tech Solutions for the first…

On Mar. 19, 2021, Scientists at the University of Oxford released pre-print data measuring the level of antibodies…

On Mar. 18, 2021, XPhyto and 3a-diagnostics announced the European approval of its point-of-care SARS-CoV-2 (COVID-19) RT-PCR test…

On Mar. 18, 2021, AstraZeneca announced that the Medicines and Healthcare products Regulatory Agency (MHRA) and European Medicines…
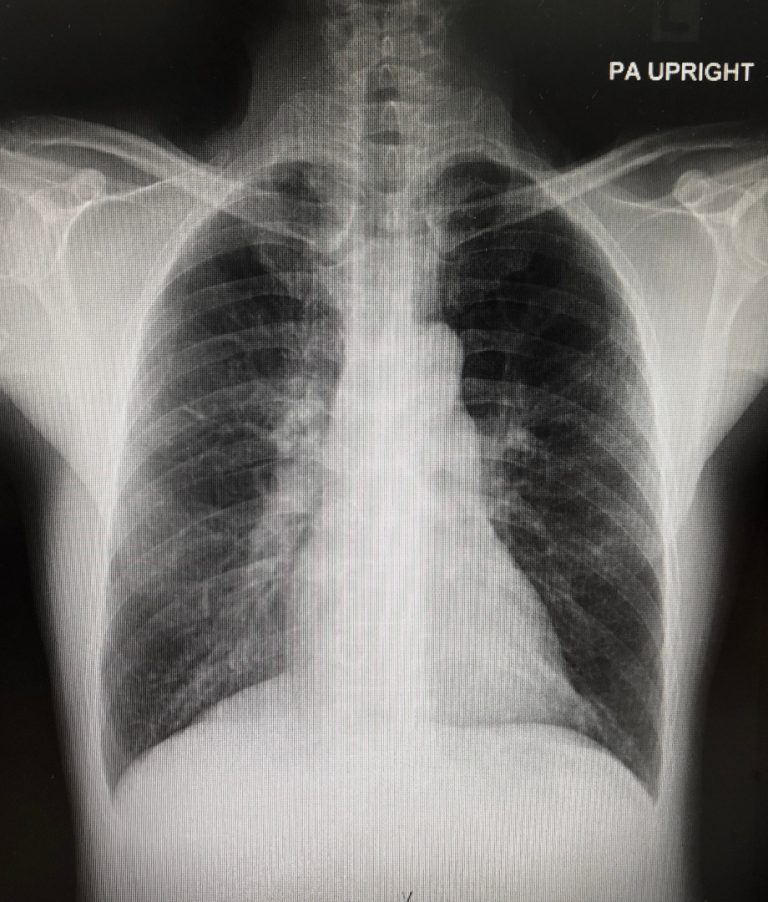
On Mar. 18, 2021, Incyte announced results from the Phase 3 DEVENT study evaluating the efficacy and safety…

On Mar. 18, 2021, the Centers for Disease Control and Prevention (CDC) announced a research initiative that offers…

On Mar. 17, 2021, the FDA granted marketing authorization of the BioFire Respiratory Panel 2.1 (RP2.1), a diagnostic…

On Mar. 17, 2021, Tonix Pharma announced preliminary results following vaccination of non-human primates with TNX-1800 (modified horsepox…

On Mar. 17, 2021, Altasciences working with the National Research Council of Canada (NRC), announced that it had…

On Mar. 17, 2021, the U.S. Department of Health and Human Services (HHS) announced the investment of $10…
On Mar. 17, 2021, the WHO reported that the AstraZeneca COVID-19 vaccine (including Covishield) continued to have a…

On Mar. 16, 2021, AstraZeneca announced it had modified an existing agreement with the US Government to supply…

On Mar. 16, 2021, U.S. military researchers announced they had found that headache, dizziness and cognitive dysfunction occur…

On Mar. 16, 2021, Roche announced the launch of the cobasᆴ SARS-CoV-2 Variant Set 1 Test to detect…
On Mar. 16, 2021, research from the WHO and partners showed that the COVID-19 pandemic was severely affecting…

On Mar. 16, 2021, TGen announced a study that suggested ecosystems suitable for harboring ticks that carry debilitating…

On Mar. 16, 2021, Moderna announced that the first participants had been dosed in the Phase 2/3 study,…

On Mar. 15, 2021, Altimmune announced additional preclinical data for its single dose intranasal COVID-19 vaccine candidate, AdCOVID….

On Mar. 15, 2021, Moderna announced that the first participants had been dosed in the Phase 1 study…

On Mar. 15, 2021, Twist Bioscience announced that it had started shipping its new synthetic RNA reference controls,…

On Mar. 15, 2021, ImmunityBio announced it had met the safety requirements for the first 12 participants in…

On Mar. 15, 2021, Resverlogix announced the publishing of a new article titled: モBromodomain and extraterminal protein inhibitor,…