RNA ‘ticker tape’ records gene activity over time
On Oct. 19, 2020, researchers at MIT, Harvard, and the Broad Institute of MIT announced they had developed…

On Oct. 19, 2020, researchers at MIT, Harvard, and the Broad Institute of MIT announced they had developed…

On Oct. 19, 2020, ICON announced that it had been re-selected by the Biomedical Advanced Research and Development…

On Oct. 19, 2020, Bio-Techne announced that ProteinSimple, a Bio-Techne brand, released its SARS-CoV-2 Multi-Antigen Serology Module for…

On Oct. 19, 2020, Atossa Therapeutics announced it had completed enrollment in its Phase 1 clinical study using…

On Oct. 19, 2020, Evotec announced that its Seattle-based subsidiary, Just – Evotec Biologics had received a grant…

On Oct. 19, 2020, LabCorp announced a test that provided a quantitative measurement of an individualメs SARS-CoV-2 IgG…

On Oct. 16, 2020, the U.S. Department of Defense in coordination with the Department of Health and Human…
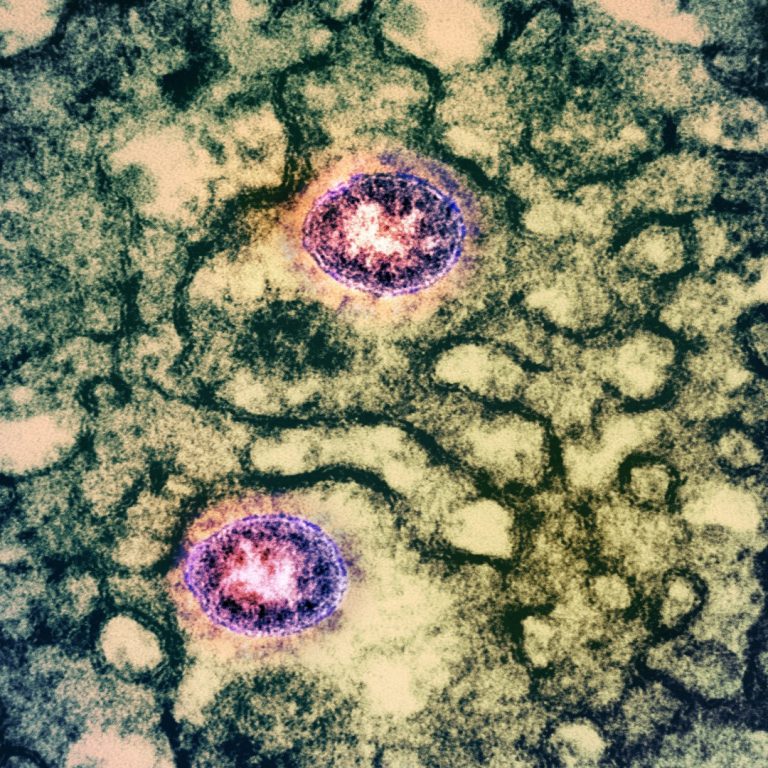
On Oct. 16, 2020, BD (Becton, Dickinson) announced the CE mark and European availability of a product for…

On Oct. 16, 2020, the NIH launched an adaptive Phase 3 clinical trial to evaluate the safety and…
On Oct. 15, 2020, scientists from the University of Oxford’s Department of Physics announced they had developed an…

On Oct. 15, 2020, Tonix Pharmaceuticals announced that the first participant was enrolled in the observational PRECISION study…

On Oct. 15, 2020, Eiger BioPharmaceuticals announced results of the ILIAD Study (Interferon Lambda for Immediate Antiviral Therapy…

On Oct. 15, 2020, ImmunityBio announced it had received authorization from the FDA to begin a Phase I…

On Oct. 15, 2020, Ionis Pharmaceuticals announced that IONIS-PKK-LRx was being evaluated in an investigator-initiated Phase 2 clinical…

On Oct. 15, 2020, Entos Pharmaceuticals announces that its San Diego-based spinout company Aegis Life, will pursuing U.S….

On Nov. 9, 2020, after more than two years of reconstruction efforts, scientists from the Fred Hutchinson Cancer…

On Oct. 15, 2020 research teams from two divisions of the Allen Institute, the Allen Institute for Cell…

On Oct. 15, 2020, the World Health Organization (WHO) announced Interim that results from the Solidarity Therapeutics Trial,…

On Oct. 14, 2020, Vaxart announced that the FDA had completed its review of the Companyメs Investigational New…

On Oct. 14, 2020, Moderna announced that it had received written confirmation from the European Medicines Agency (EMA)…
On Oct. 14, 2020, the World Health Organization (WHO) announced that prior to the COVID-19 pandemic, many countries…
On Oct. 14, 2020, the FDA approved Regeneron Pharmaceutical’s Inmazeb (atoltivimab, maftivimab, and odesivimab-ebgn), a mixture of three…

On Oct. 14, 2020, the U.S. Department of Health and Human Services (HHS) and Department of Defense (DOD)…
On Oct. 14, 2020, recognizing the urgent need for new tools to combat vector-borne diseases (VBDs), and in…

On Oct. 14, 2020, 3M and Discovery Education announced they had named 14-year-old Anika Chebrolu from Frisco, Texas,…
On Oct. 14, 2020, Regeneron announced that the FDA had approved Inmazeb (atoltivimab, maftivimab and odesivimab-ebgn) for the…

On Oct. 14, 2020, Thermo Fisher Scientific announced plans to develop two new sterile filling lines in Singapore…

On Oct. 14, 2020, Tevogen Bio announced that its Investigational New Drug (IND) application to develop a COVID-19…

On Oct. 14, 2020, Pfizer and BioNTech announced that preliminary, peer-reviewed data from the Phase 1 portion of…

On Oct. 14, 2020, Sorrento Therapeutics announced receipt of clearance from the Brazilian regulatory agency (ANVISA) to proceed…