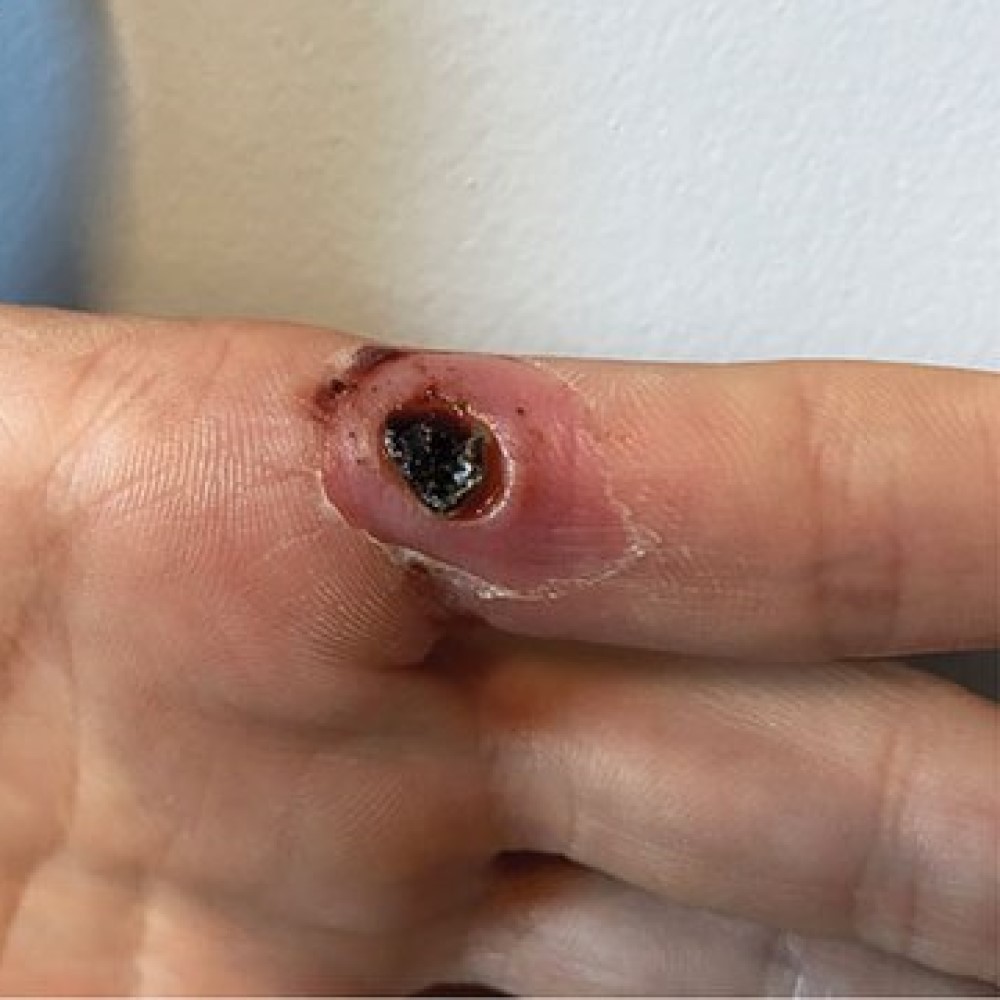
Emergent BioSolutions’ ACAM2000® Received U.S. FDA Approval for Mpox Indication
On Aug. 29, 2024, Emergent BioSolutions announced that the U.S. Food and Drug Administration (FDA) had approved the supplemental Biologics License Application (sBLA) for the expansion of the indication for ACAM2000®, (Smallpox and Mpox (Vaccinia) Vaccine, Live) to include prevention of mpox disease in individuals determined to be at high risk for mpox infection.
The approval was based on previously available human safety data and data from a well-controlled animal study in which ACAM2000® vaccine was shown to be effective in protecting against mpox virus exposure. The vaccine was first approved by the FDA in 2007 for active immunization for the prevention of smallpox disease in individuals determined to be at high risk for smallpox infection.
Tags:
Source: Emergent BioSolutions
Credit:
