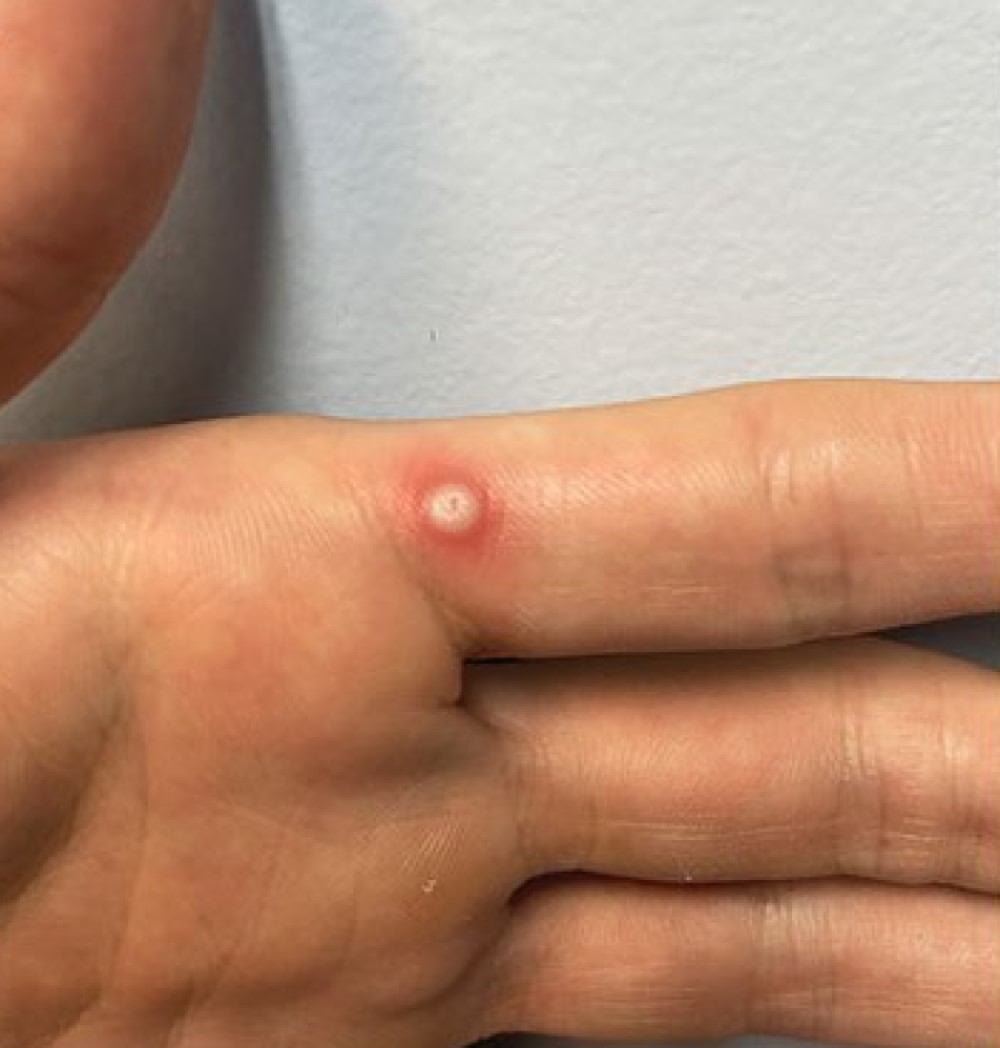
WHO approved first mpox diagnostic test for emergency use, boosting global access
On Oct. 3, 2024, the World Health Organization (WHO) announced it had listed the first mpox in vitro diagnostic (IVD) under its Emergency Use Listing (EUL) procedure, an important step in improving global access to mpox testing.
The approval for emergency use of the Alinity m MPXV assay, manufactured by Abbott Molecular, will be pivotal in expanding diagnostic capacity in countries facing mpox outbreaks, where the need for quick and accurate testing has risen sharply. Early diagnosis of mpox enables timely treatment and care, and control of the virus.
Tags:
Source: World Health Organization
Credit:
