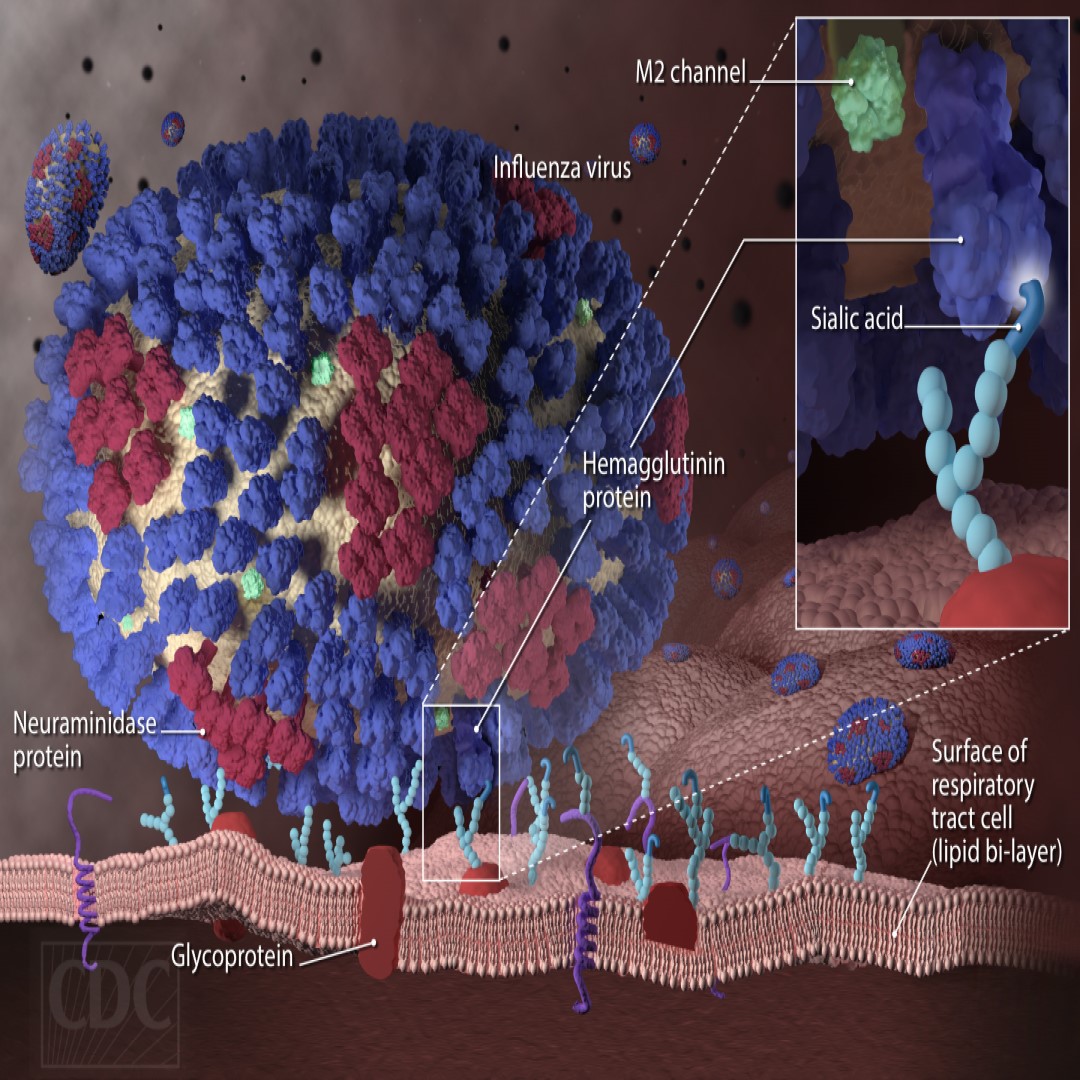
Vaccitech universal flu vaccine passed Phase 2b clinical development milestones
On Jun. 5, 2019, Vaccitech announced that it has administered its pandemic universal influenza A vaccine MVA-NP+M1 (VTP-100) to the first participants in a Flu 010 study – a Phase 2b, randomised, double-blind, placebo controlled, influenza challenge study being conducted in Antwerp, Belgium.
Tags:
Source: Vaccitech
Credit:
