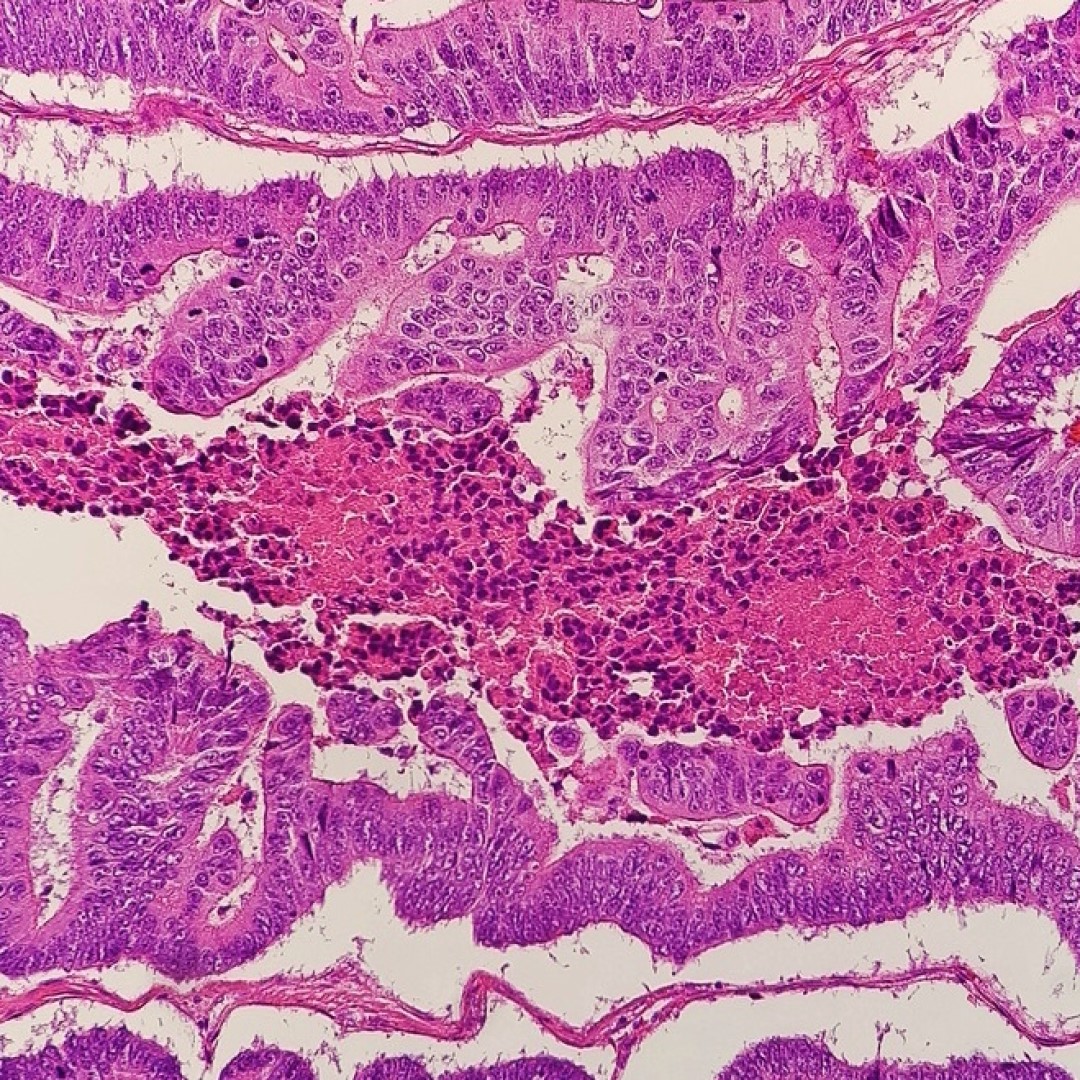
The monoclonal antibody cetuximab (Erbitux) was approved by the FDA for the treatment of metastatic colorectal cancer
On Feb. 1, 2004, the U.S. Food and Drug Administration (FDA) announced it had approved Cetuximab, a recombinant human/mouse chimeric epidermal growth factor receptor (EGFR) monoclonal antibody, to be used in combination with irinotecan for the treatment of EGFR-expressing, metastatic colorectal cancer in patients who had failed to improve with irinotecan-based chemotherapy.
Cetuximab was also approved for administration as a single agent in the treatment of patients with EGFR-expressing, metastatic colorectal cancer who are intolerant to irinotecan-based chemotherapy.
Tags:
Source: U.S. National Library of Medicine
Credit: Photo: Micrograph of a moderately differentiated colorectal carcinoma with dirty necrosis. Courtesy: Mikael Häggström, M.D.
