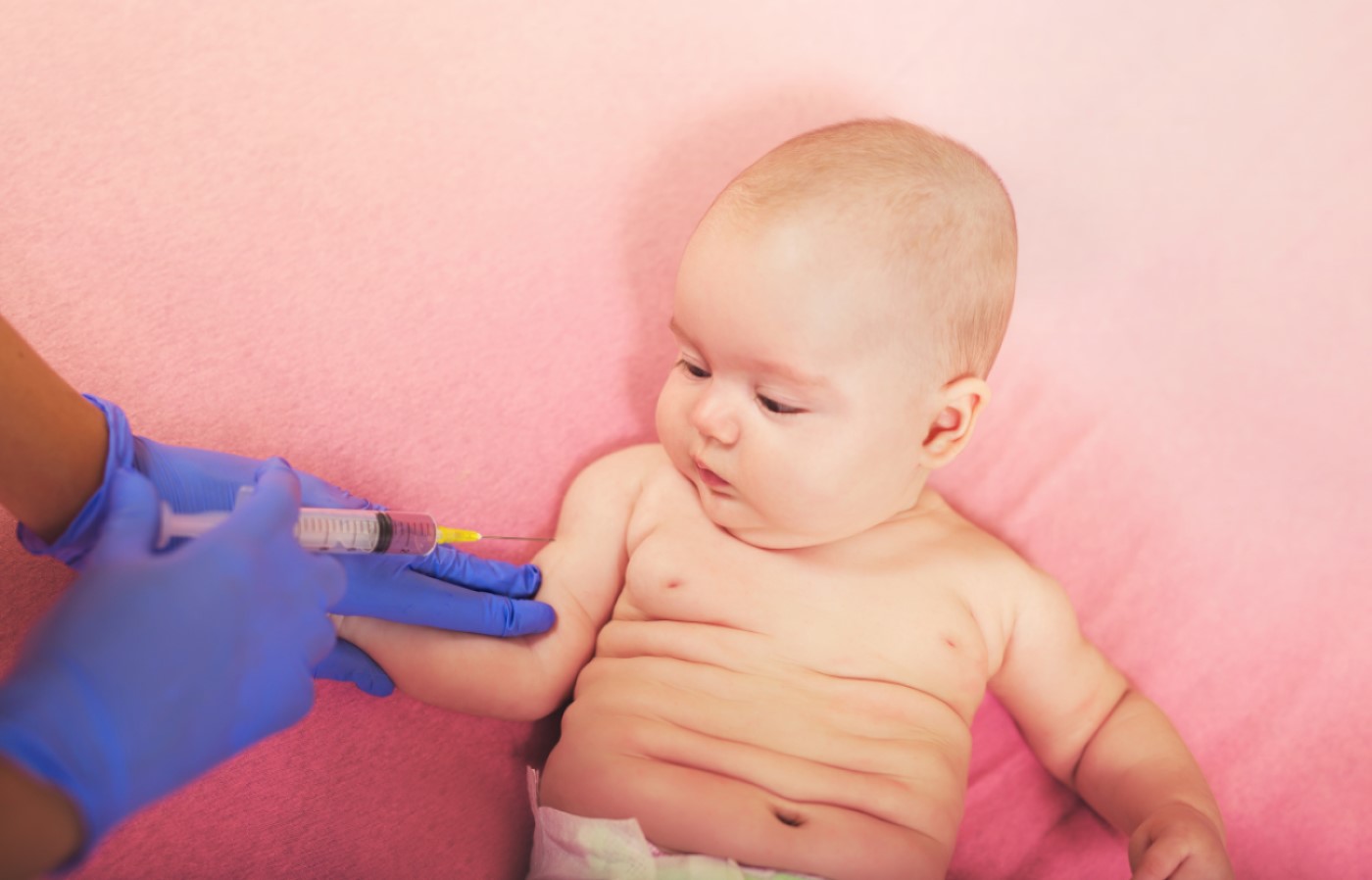
The FDA approved use of a new haemophilus b conjugate vaccine for infants and children
On Mar. 30, 1993, the U.S. Food and Drug Administration (FDA) approved a new Haemophilus b conjugate vaccine, polyribosylribitol phosphate-tetanus toxoid conjugate (PRP-T), manufactured by Pasteur Merieux Serum et Vaccins and distributed as ActHIB Trademark by Connaught Laboratories (Swiftwater, Pennsylvania).
This vaccine was licensed for use in infants in a three-dose primary vaccination series administered at ages 2, 4, and 6 months. Previously unvaccinated infants 7-11 months of age should receive two doses 2 months apart. Previously unvaccinated children 12-14 months of age should receive one dose. A booster dose administered at 15 months of age is recommended for all children. Previously unvaccinated children 15-59 months of age should receive a single dose and do not require a booster.
Tags:
Source: U.S. Centers for Disease Control and Prevention
Credit:
