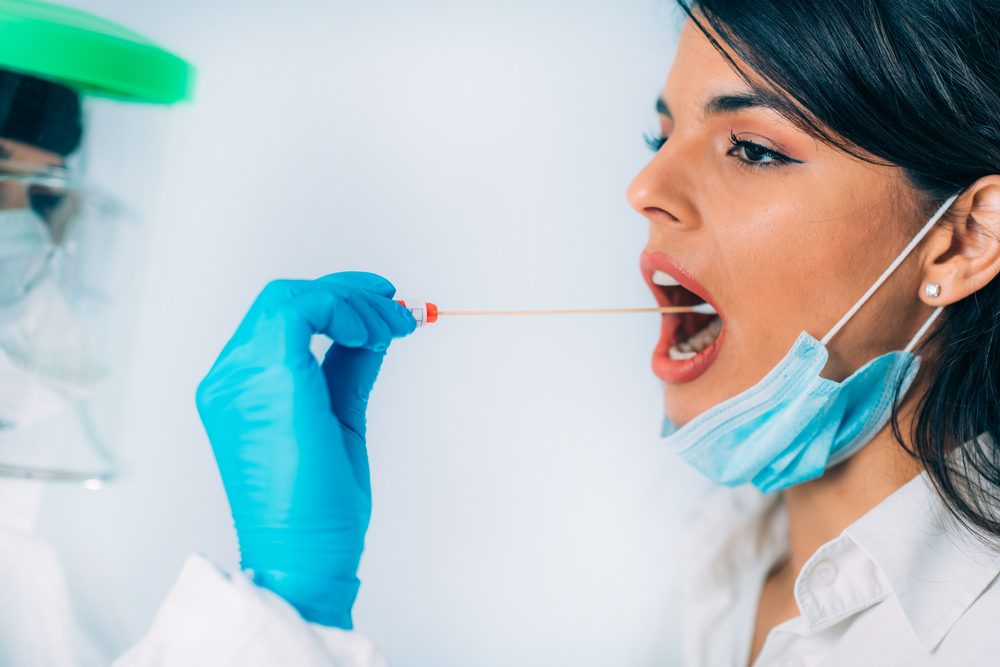
The FDA approved the first US HIV test system using oral fluid samples
On Dec. 23, 1994, the FDA announced the approval of the first U.S. HIV test system using oral fluid samples. Previously, all approved HIV tests used blood samples. In approving the new HIV test system, the FDA approved both a product for collecting specimens of oral fluid and a specific test used to analyze the specimens for the presence of antibodies to HIV, the virus that causes AIDS.
The test system is intended for use in subjects 13 years of age or older. Use of the specimen collection product is subject to several restrictions.
The oral fluid sample collection kit for HIV testing was manufactured by Epitope of Beaverton, OR, and was marketed under the name “OraSure HIV-1 Oral Specimen Collection Device.” The Oral Fluid Vironostika HIV-1 Microelisa System approved to test specimens was manufactured by Organon Teknika Corporation of Durham, N.C.
Tags:
Source: United Press International
Credit:
