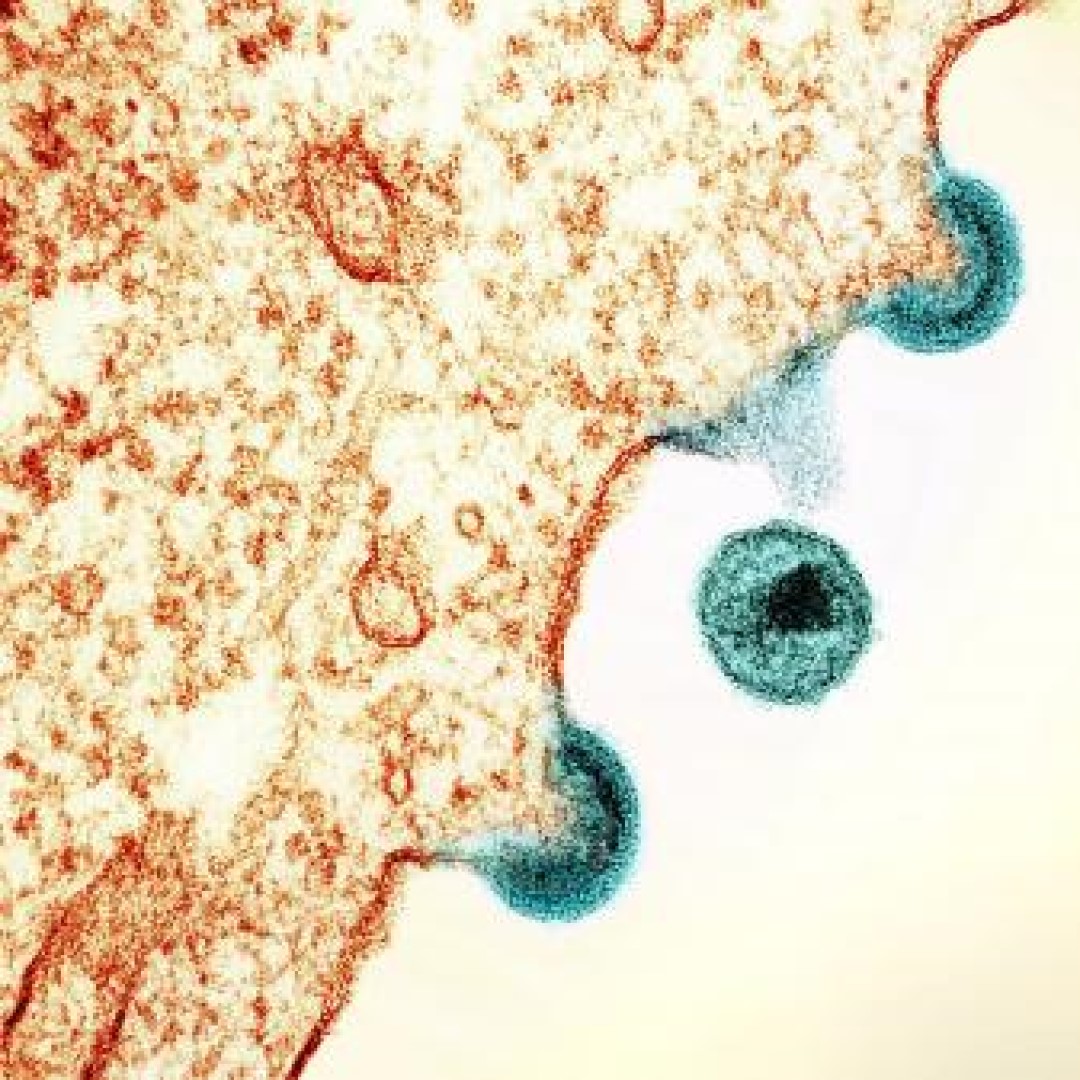
The FDA approved new HIV treatment for patients with limited treatment options
On Jul. 2, 2020, the U.S. Food and Drug Administration (FDA) approved Rukobia (fostemsavir), a new type of antiretroviral medication for adults living with HIV who have tried multiple HIV medications and whose HIV infection cannot be successfully treated with other therapies because of resistance, intolerance or safety considerations. The FDA granted approval of Rukobia to ViiV Healthcare.
Tags:
Source: U.S. Food and Drug Administration
Credit:
