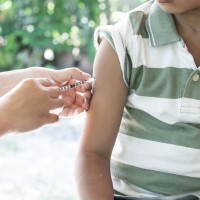
Pfizer and BioNTech announced positive topline results from pivotal trial of COVID-19 vaccine in children 5 to 11 years
On Sept. 20, 2021, Pfizer and BioNTech announced results from a Phase 2/3 trial showing a favorable safety profile and robust neutralizing antibody responses in children 5 to 11 years of age using a two-dose regimen of 10 ᄉg administered 21 days apart, a smaller dose than the 30 ᄉg dose used for people 12 and older.
The antibody responses in the participants given 10 ᄉg doses were comparable to those recorded in a previous Pfizer-BioNTech study in people 16 to 25 years of age immunized with 30 ᄉg doses. These were the first results from a pivotal trial of a COVID-19 vaccine in this age group.
Tags:
Source: BioNTech
Credit:
