NIH clinical trial of remdesivir to treat COVID-19 began for adults with coronavirus disease
On Feb. 25, 2020, the National Institutes of Health (NIH) announced that a randomized, controlled clinical trial to evaluate the safety and efficacy of the investigational antiviral remdesivir in hospitalized adults diagnosed with coronavirus disease 2019 (COVID-19) had begun at the University of Nebraska Medical Center in Omaha.
This was the first clinical trial in the U.S. to evaluate an experimental treatment for COVID-19, the respiratory disease first detected in December 2019 in Wuhan, Hubei Province, China. remdesivir, developed by Gilead Sciences, was an investigational broad-spectrum antiviral treatment.
Tags:
Source: U.S. National Institutes of Health
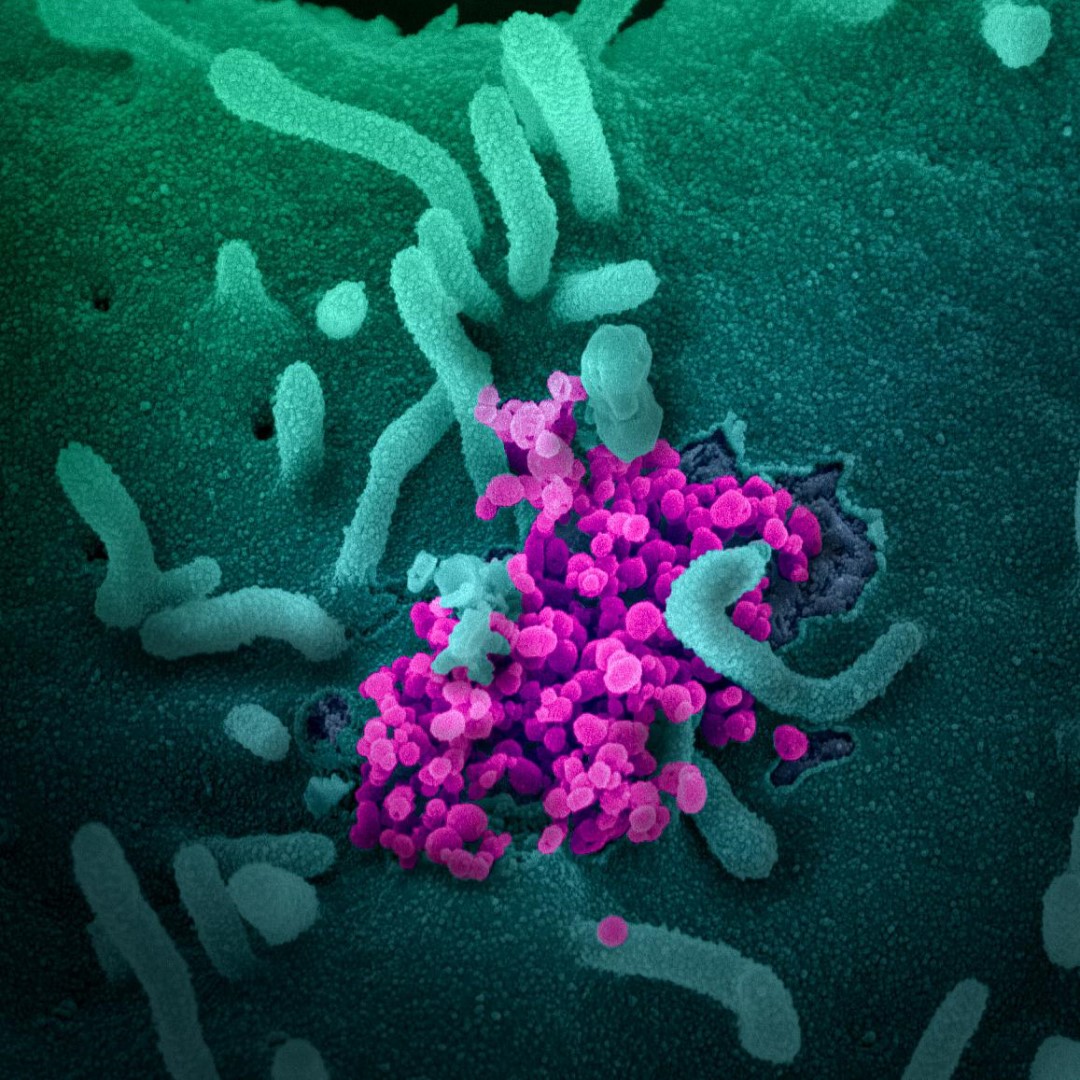
Credit: Scanning electron microscope image show SARS-CoV-2 SARS-CoV-2 (round magenta objects) emerging from the surface of cells cultured in the lab. SARS-CoV-2, also known as 2019-nCoV, is the virus that causes COVID-19. The virus shown was isolated from a patient in the U.S. Image Courtesy: NIAID-RML.
