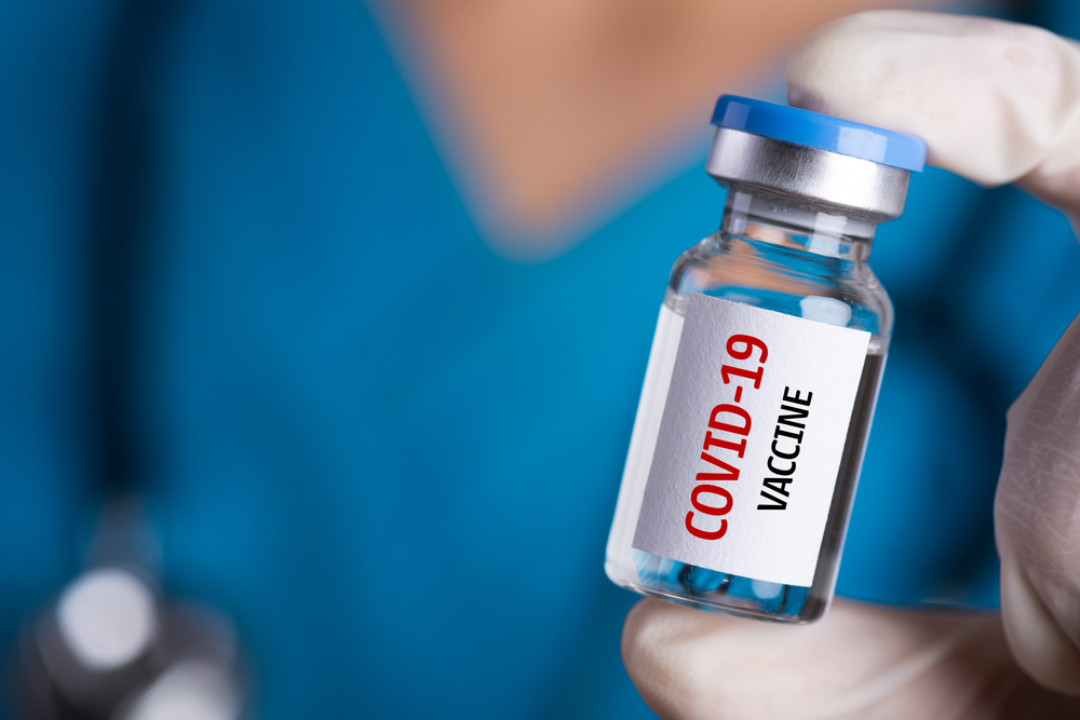
Moderna announced FDA authorization of Moderna COVID-19 vaccine in U.S.
On Dec. 18, 2020, Moderna announced that the U.S. Food and Drug Administration (FDA) had authorized the emergency use of mRNA-1273, Moderna’s vaccine against COVID-19 in individuals 18 years of age or older. The Moderna COVID-19 Vaccine was authorized for distribution and use under an Emergency Use Authorization (EUA).
Delivery to the U.S. Government began immediately following authorization. Moderna continued to gather additional data and plans to file a Biologics License Application with the FDA requesting full licensure in 2021.
Tags:
Source: U.S. Food and Drug Administration
Credit:
