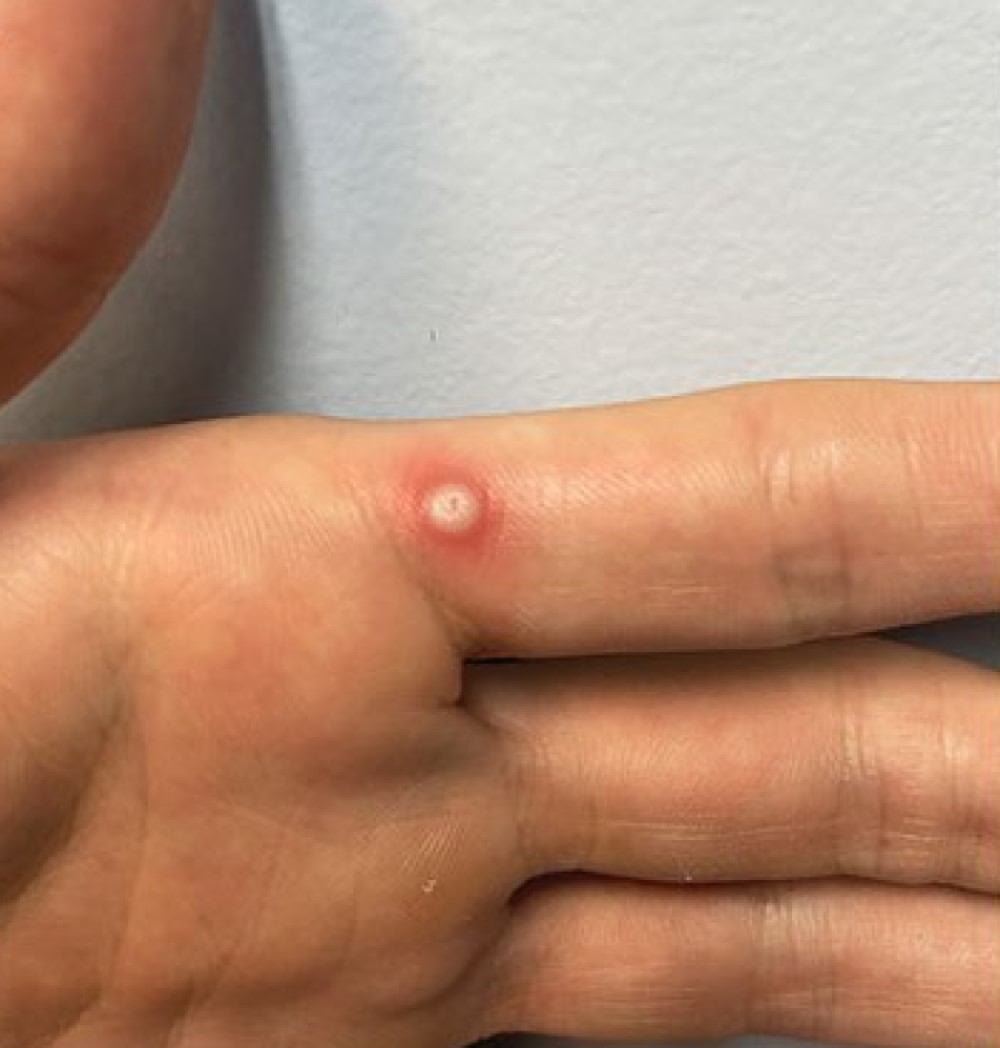
Labcorp received FDA Emergency Use Authorization for Mpox PCR test home collection kit
On Apr. 10, 2024, LabCorp announced that the U.S. Food and Drug Administration (FDA) had granted Emergency Use Authorization (EUA) for its Mpox PCR Test Home Collection Kit to aid in the diagnosis of infection with non-variola Orthopoxvirus, including the monkeypox virus that causes monkeypox, also known as mpox.
The test is the first mpox at-home collection kit authorized by FDA and is available to physicians to order for patients 18 years of age or older who are suspected of mpox infection.
Physicians can order a test through Labcorp’s provider interface platform for patients they suspect may be infected with the virus. Labcorp will send the test kit directly to patients for at-home collection. The kit includes detailed instructions for patients on correctly collecting a lesion swab, securing the sample in the provided collection tube, and preparing the package for return to an authorized laboratory for analysis.
Testing of specimens collected using the test kit will employ PCR (polymerase chain reaction) technology and will be conducted in authorized laboratories designated by Labcorp and certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA) to meet the requirements for performing high-complexity testing criteria for the detection of non-variola Orthopoxvirus DNA. Results are electronically delivered to the prescribing physician and made available to the patient in Labcorp’s patient portal at patient.labcorp.com. The company also aims to make the test available on its Labcorp OnDemand platform.
The authorization comes amid reported increases in mpox cases in the United States. According to the Centers for Disease Control and Prevention (CDC), there have been 511 mpox cases reported in 2024 through March 16, compared to fewer than 300 cases reported by late March 2023. Since the onset of the national 2022-2023 mpox virus outbreak, part of a larger global outbreak of human mpox caused by the West African clade of the monkeypox virus, the CDC notes more than 32,000 cases and 58 deaths have been reported nationally.
Tags:
Source: LabCorp
Credit:
