Genentech drug Venclexta (venetoclax) was approved
On Apr. 11, 2016, the U.S. Food and Drug Administration (FDA) announced that it had granted accelerated approval to VenclextaTM (venetoclax) for the treatment of people with chronic lymphocytic leukemia (CLL) with 17p deletion, as detected by an FDA approved test, who have received at least one prior therapy.
Venclexta was the first approved medicine designed to help restore a process in which cells self-destruct (apoptosis) by selectively blocking the BCL-2 protein and is Genentech’s tenth new medicine approved in the past seven years. Venclexta was being developed by AbbVie and Genentech.
Tags:
Source: Genentech
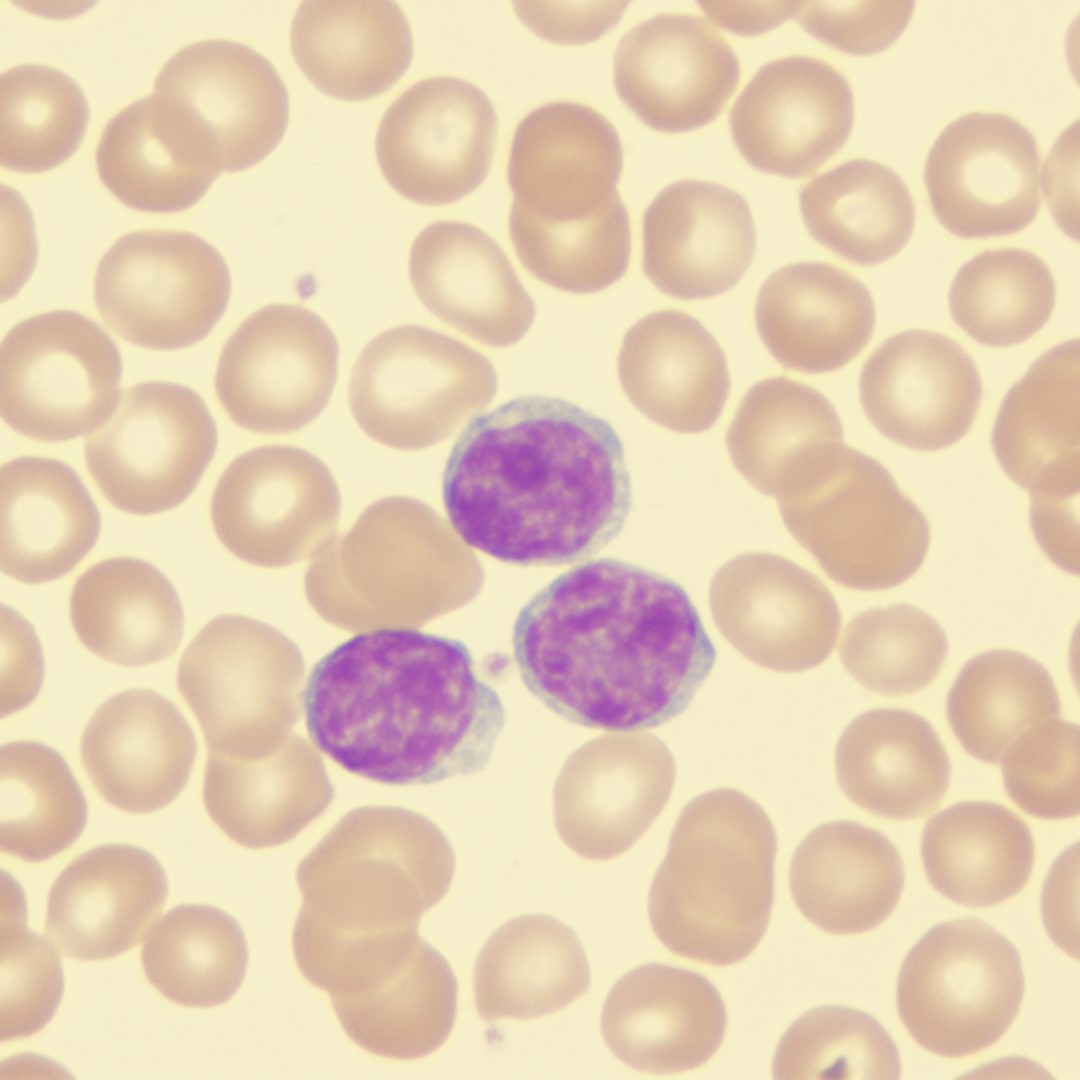
Credit: Photo: Microscopic image of Wright’s stained peripheral blood smear showing chronic lymphocytic leukemia (CLL) with the darkly staining nuclei. Courtesy: Wikipedia.
