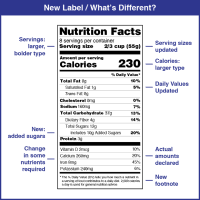
FDA revised the Nutrition and Supplement Facts Labels
On May 27, 2016, the Food and Drug Administration (FDA) announced it had amended its labeling regulations for conventional foods and dietary supplements to provide updated nutrition information on the label to assist consumers in maintaining healthy dietary practices.
The compliance date of this final rule is July 26, 2018 for manufacturers with $10 million or more in annual food sales and July 26, 2019 for manufacturers with less than $10 million in annual food sales.
Tags:
Source: Federal Register
Credit:
