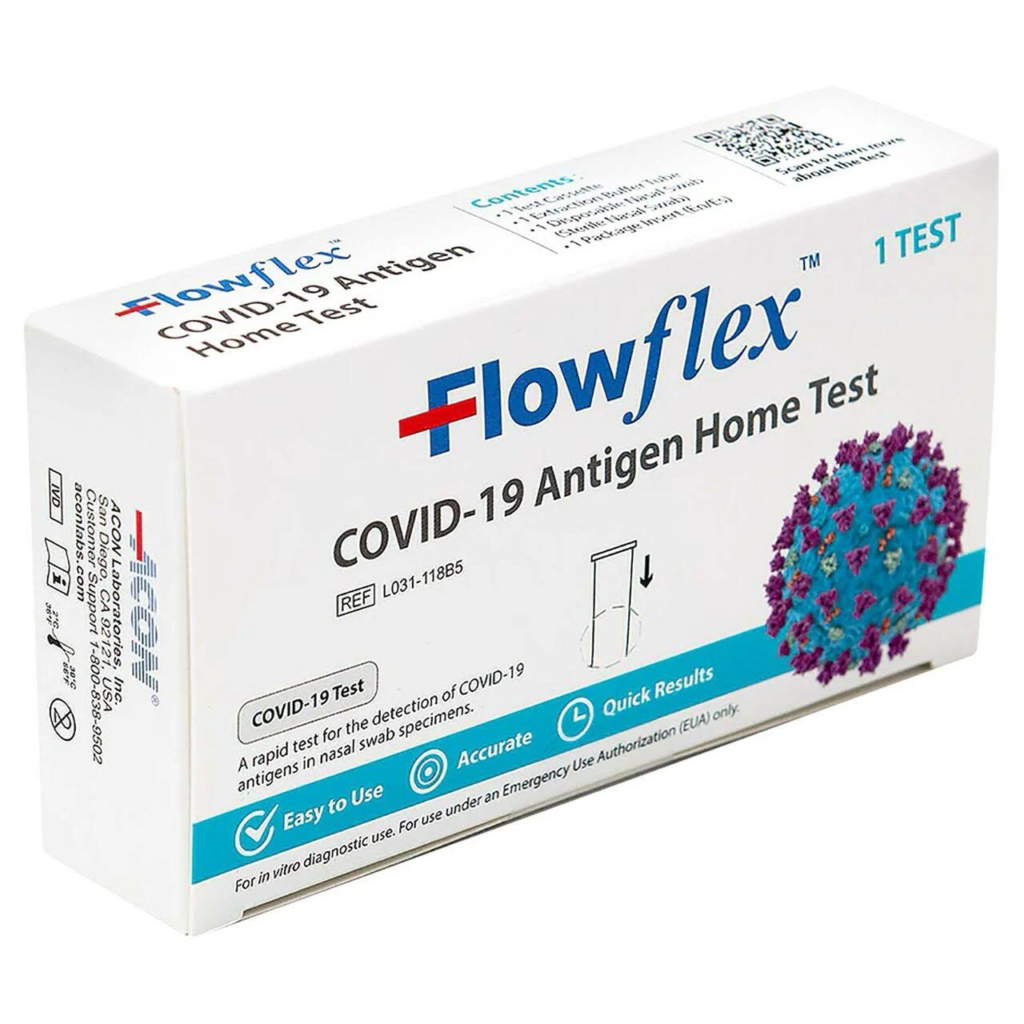
FDA cleared first COVID-19 home antigen test
On Nov. 9, 2023, the U.S. Food and Drug Administration (FDA) cleared for marketing the first over-the-counter (OTC) antigen test for COVID-19. ACON Laboratories’ Flowflex COVID-19 Antigen Home Test, originally authorized for emergency use in 2021, is now the second home COVID-19 test to successfully complete a traditional FDA premarket review pathway, and the first indicated for use in children under 18.
The Flowflex COVID-19 Antigen Home Test is a visually-read test cleared for OTC home use by symptomatic individuals within six days of symptom onset. It is cleared for individuals aged 14 years or older testing themselves, or adults testing individuals aged two years or older. In a study reviewed by the FDA, this test correctly identified 89.8% of positive and 99.3% of negative samples in individuals with signs and symptoms of upper respiratory infection.
As with antigen tests authorized for emergency use, this test is intended to be used at least twice over three days with at least 48 hours between tests. This means that a symptomatic individual with an initial negative test result should be re-tested once between 48 and 72 hours after the first test using an antigen test for COVID-19 or followed up with a molecular COVID-19 test.
The FDA reviewed the ACON Flowflex COVID-19 Antigen Home Test through the 510(k) premarket review pathway. A 510(k) is a premarket submission made to the FDA to demonstrate that a new device is substantially equivalent to a legally marketed predicate device.
Tags:
Source: U.S. Food and Drug Administration
Credit:
