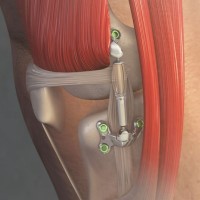
FDA authorizes marketing of MISHAル Knee System for people Suffering from knee osteoarthritis
On Apr. 10, 2023, the U.S. Food and Drug Administration authorized for marketing Moximed’s MISHA Knee System, an implant placed alongside the knee joint to help reduce the amount of load carried by part of the joint. Unlike conventional knee replacement, the knee joint is not removed with this device.
The MISHA Knee System is indicated for patients with osteoarthritis of certain parts of the knee who have failed to find relief with surgical or non-surgical treatment and are still experiencing pain that interferes with activities of daily living. These patients are also unwilling to undergo or are ineligible for total knee replacement due to age or absence of advanced osteoarthritis.
Tags:
Source: U.S. Food and Drug Administration
Credit:
