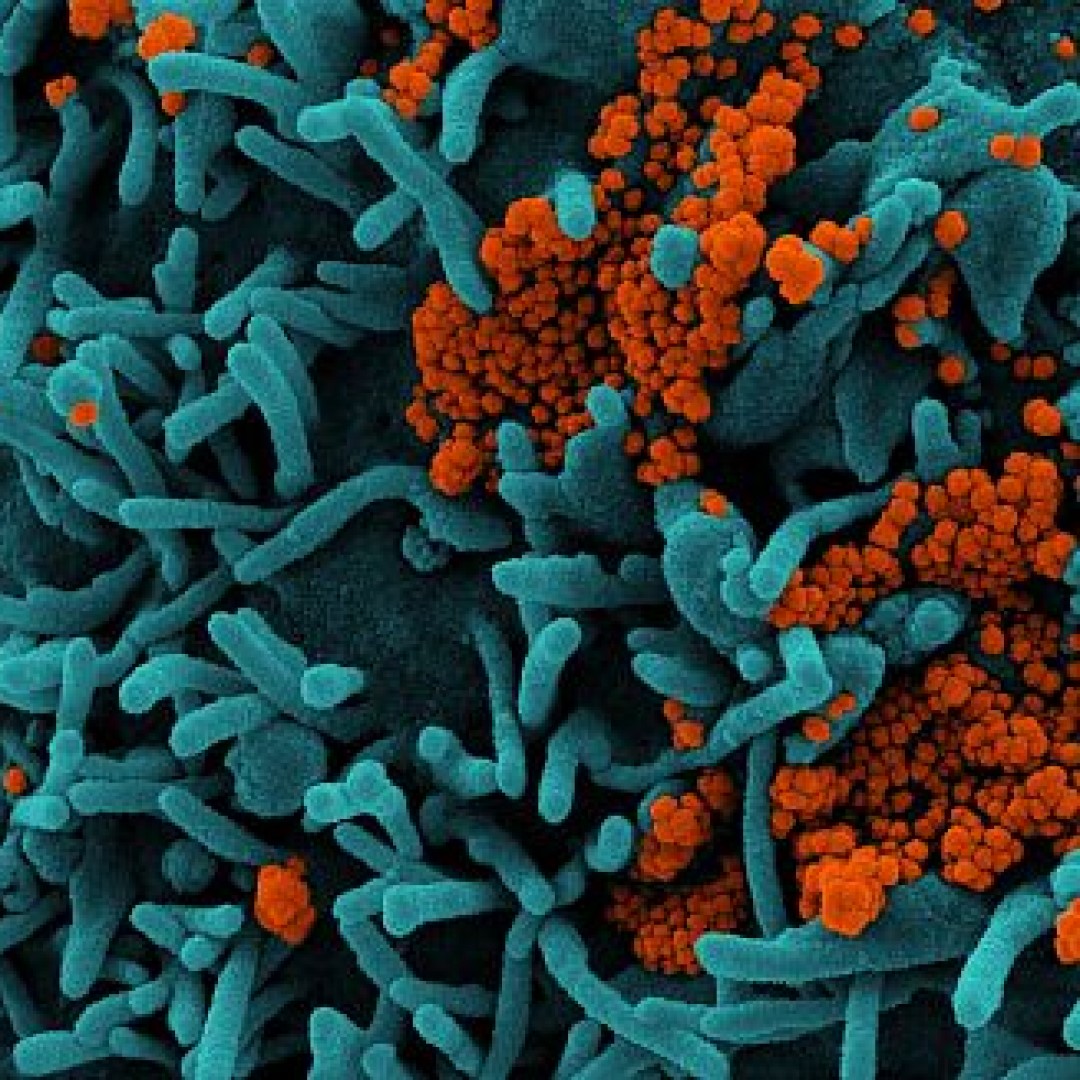
Cumberland Pharma expanded availability of Vaprisol to treat Hyponatremia for critical care COVID-19 patients
On Apr. 7, 2020, Cumberland Pharma announced an initiative to increase availability of Vaprisol (conivaptan hydrochloride) injection for treating hyponatremia, associated with critical care patients during the COVID-19 pandemic.
Vaprisol is an U.S. Food and Drug Administration (FDA) approved treatment for hyponatremia, a potentially life-threatening condition that can often afflict patients in the Intensive Care Unit
Tags:
Source: Cumberland Pharmaceuticals
Credit:
