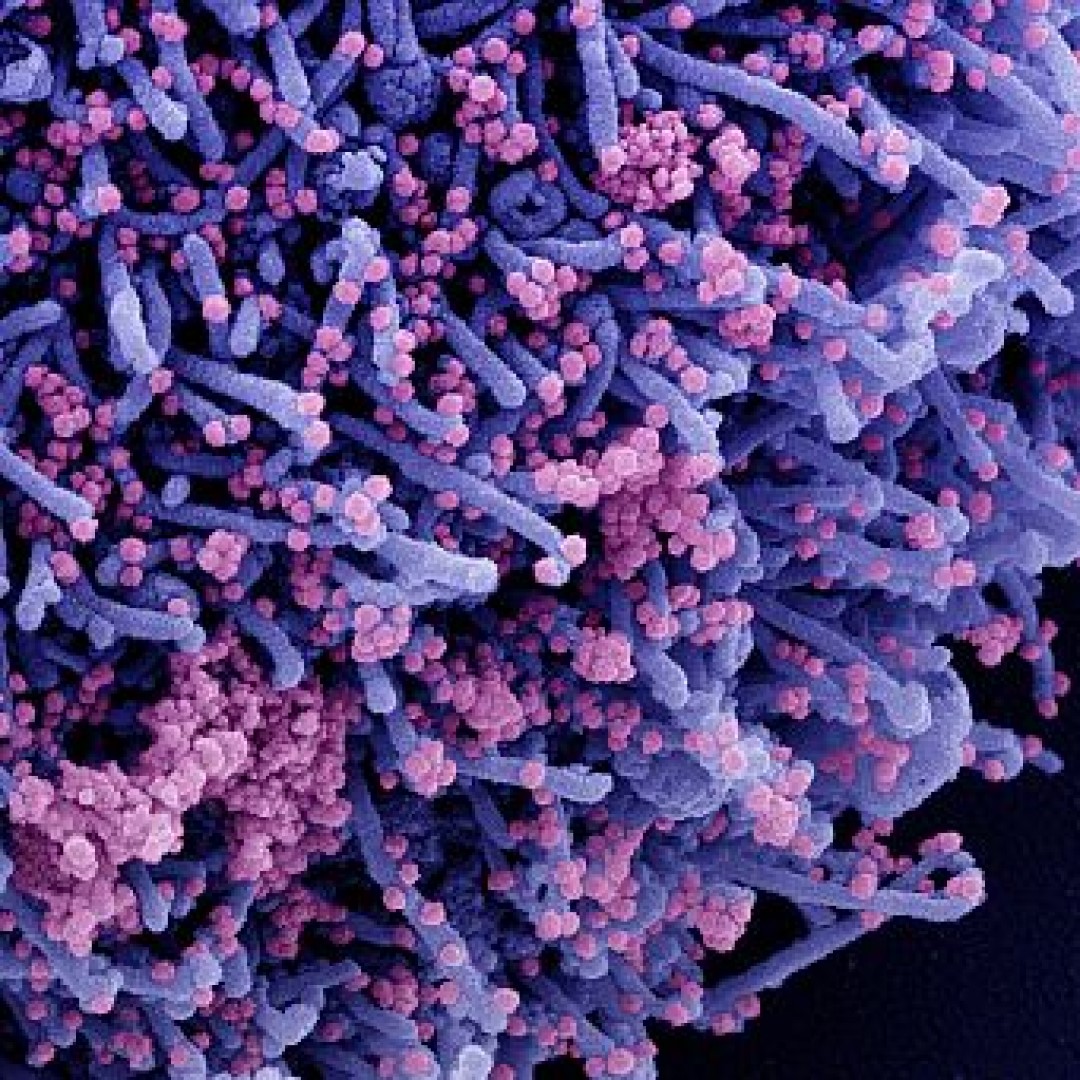
Adaptive Biotechnologies announced FDA EUA for T-Detect COVID to confirm prior COVID-19 infection
On Mar. 5, 2020, Adaptive Biotechnologies announced that the U.S. Food and Drug Administration (FDA) had issued an Emergency Use Authorization (EUA) for T-Detect COVID to confirm recent or prior COVID-19 infection.
This first-in-class T cell- based test was the first indication resulting from Adaptive’s TCR-Antigen Map collaboration with Microsoft.
Tags:
Source: Adaptive Biotechnologies
Credit:
