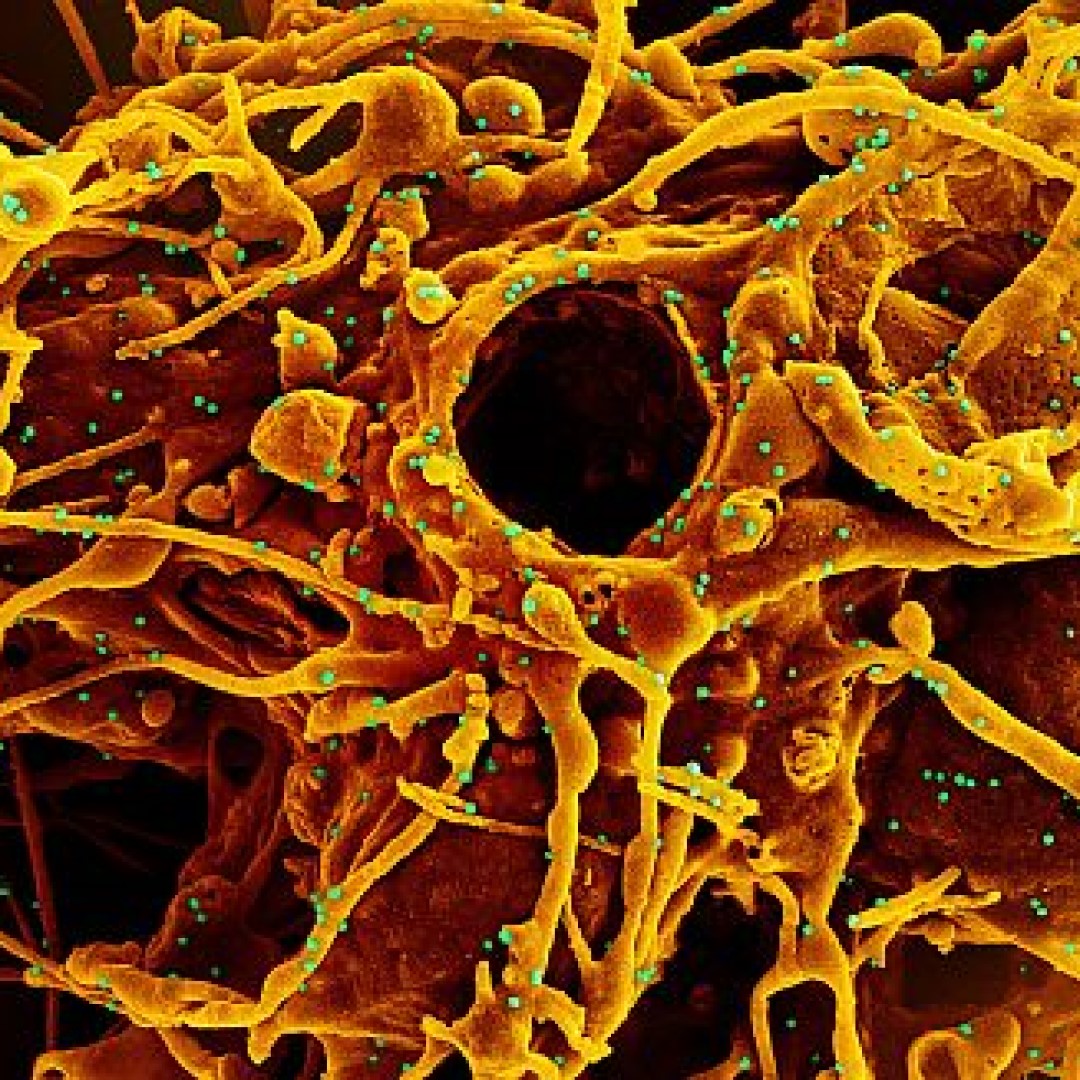
Abbott’s released ID NOW COVID-19 interim clinical study results to provide facts on clinical performance
On Oct. 7, 2020, in a continuing effort to provide the facts about ID NOW to support public health interests, Abbott shared new interim clinical data results on its ID NOW COVID-19 rapid test. The results confirmed the data submitted to the FDA in March for Emergency Use Authorization and the interim results that Abbott shared in its May 21 press release.
Tags:
Source: PR Newswire
Credit:
