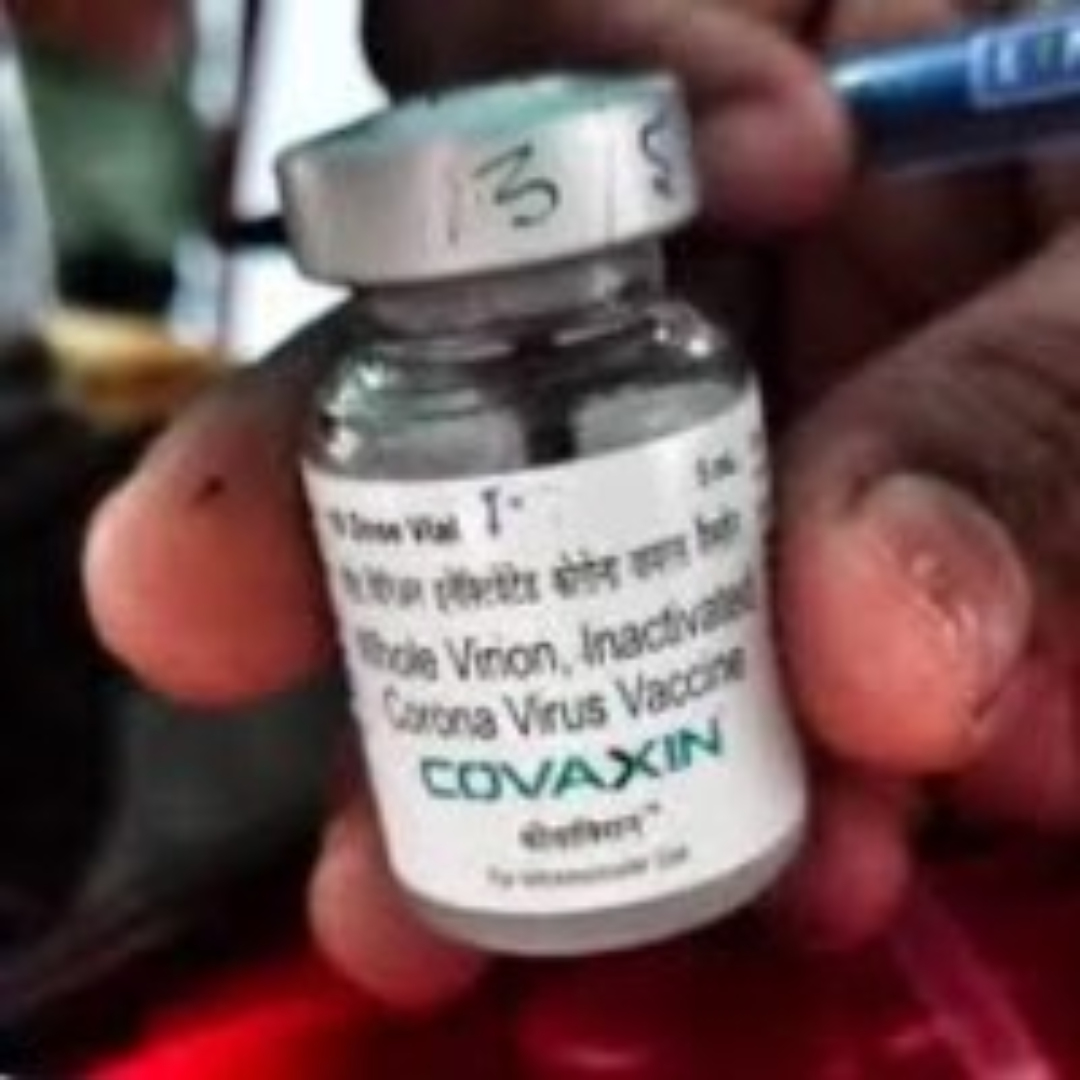
ViroVax licensed adjuvant to be used in India’s first COVID-19 vaccine in human trials
On Sept. 28 2020, ViroVax announced that it had licensed its adjuvant, Alhydroxiquim-II, to Bharat Biotech International, an Indian biotech company. Bharat Biotech partnered with the Indian Council of Medical Research (ICMR) to produce India’s first COVID-19 vaccine approved for human trials, COVAXIN, which uses ViroVaxメ\’s adjuvant technology.
Tags:
Source: ViroVax
Credit:
