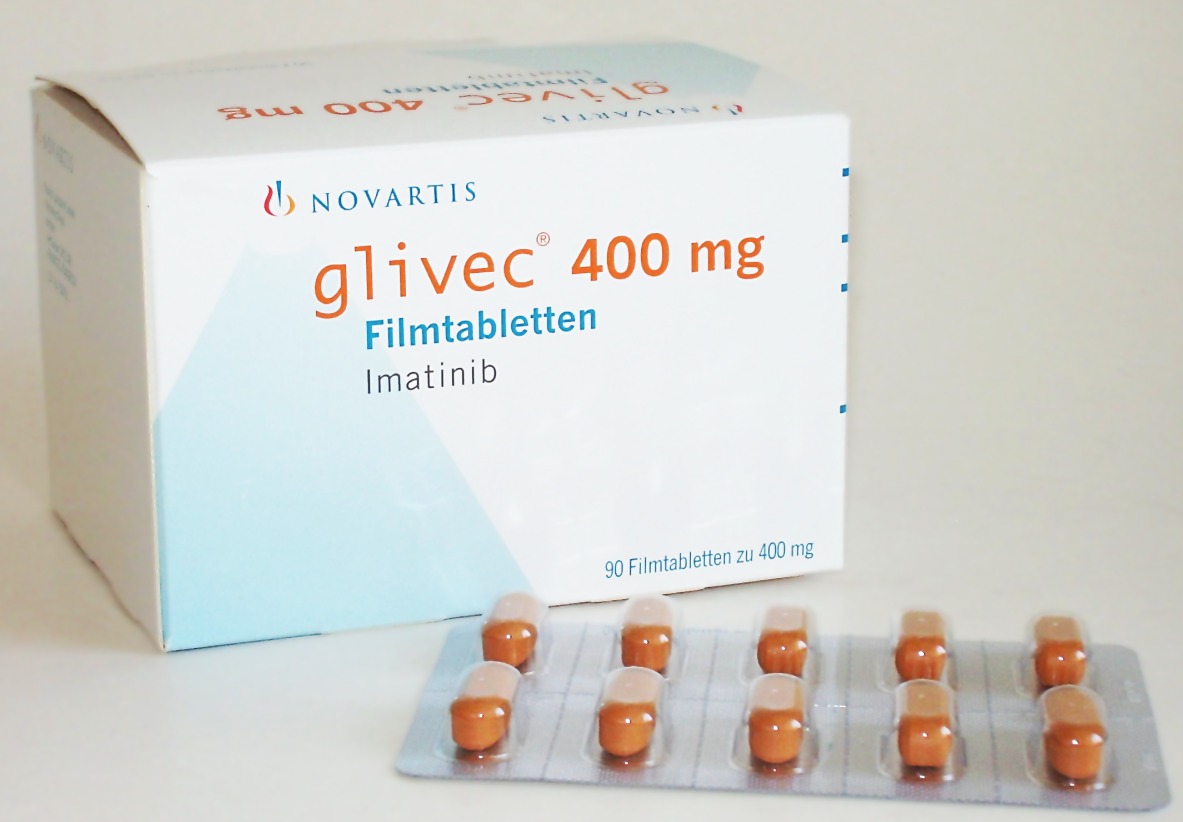
The FDA approved Gleevec, developed by OHSU, for treatment of chronic myeloid leukemia
On May 10, 2001, the U.S. Food and Drug Administration (FDA) approved Gleevec, the world’s first targeted cancer therapy in record time for treatment of chronic myeloid leukemia.
The bright orange pill, formerly known as STI571, was developed by Novartis in collaboration with Brian Druker, M.D., director of the Oregon Cancer Institute’s Leukemia Center at Oregon Health & Science University. Gleevec was later approved for nine more cancers.
The FDA later expanded the approved uses, including for nine approved orphan drug indications, greatly increasing the number of patients using the drug. Among the new indications were approvals for the treatment of gastrointestinal stromal tumors (GIST).
Tags:
Source: Harvard University
Credit:
