Chemistry-Powered “Breathing” Membrane Opens and Closes Tiny Pores on Its Own
On Feb. 18, 2026, researchers at SANKEN, University of Osaka announced using a nanoreactor to produce pores that mimic biological ion channels. Ion channels are narrow passageways that play a pivotal role in many biological processes.
To model how ions move through these tight spaces, pores need to be fabricated at very small length scales. The narrowest regions of ion channels can be just a few angstroms wide, about the size of individual atoms, making reproducible and precise fabrication a major challenge in modern nanotechnology.
In a study published in Nature Communications, researchers at The University of Osaka have addressed this challenge by using a miniature electrochemical reactor to create ultra-small pores approaching subnanometer dimensions.
In biological cells, ions flow in and out through channels in cell membranes. This ion flow is the basis for generating electrical signals, such as nerve impulses that trigger muscle contraction. The channels themselves are made of proteins and can have angstrom-wide narrow regions. Conformational changes of these proteins in response to external stimuli open and close the channels.
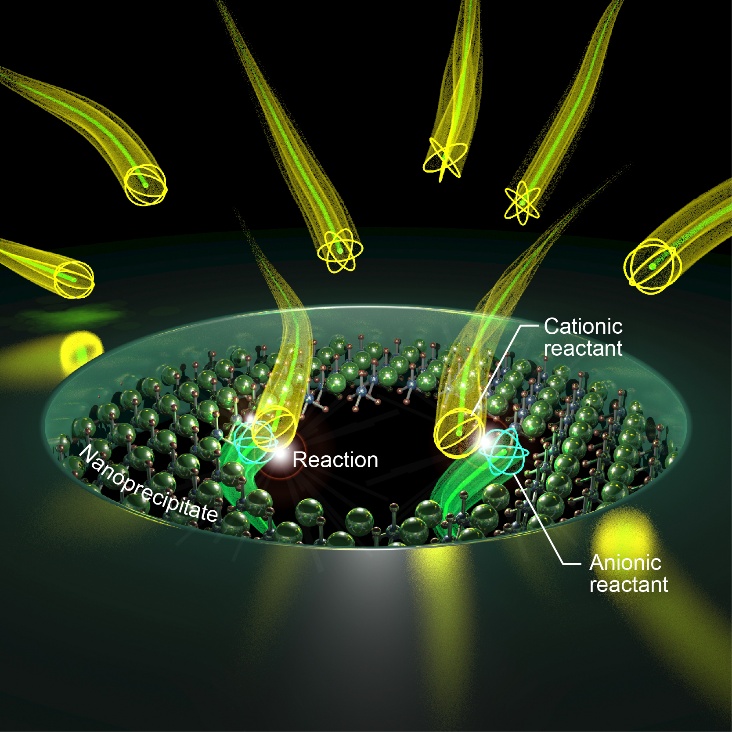
Inspired by these biological systems, the research team developed a solid-state analogue that enables the formation of subnanometer pores approaching biological ion-channel dimensions. A nanoscale pore was used as a reactor to form the subnanometer pores. First, the nanopore was created in a silicon nitride membrane. Applying a negative voltage across the membrane induced a reaction in the pore that produced a precipitate. The precipitate grew until the pore was completely blocked. Applying a positive voltage to the membrane caused the precipitate to dissolve and reopened conductive pathways within the pore.
The team measured the ion current through the membrane. There were spikes in the current, which have also been observed in biological channels. Analysis of these spikes suggests that precipitate formation is most consistent with the formation of many subnanometer pores within the nanopore.
“We were able to vary the behavior and effective size of the ultrasmall pores by changing the composition and pH of the reactant solutions,” reports Tomoji Kawai, senior author. “This enabled selective transport of ions of different effective sizes through the membrane by tuning the ultrasmall pore sizes.”
Tags:
Source: University of Osaka
Credit: Image: Schematic model depicting in-pore precipitation reaction in a solid-state nanopore. Courtesy: Credit: Makusu Tsutsui, SANKEN, University of Osaka.
