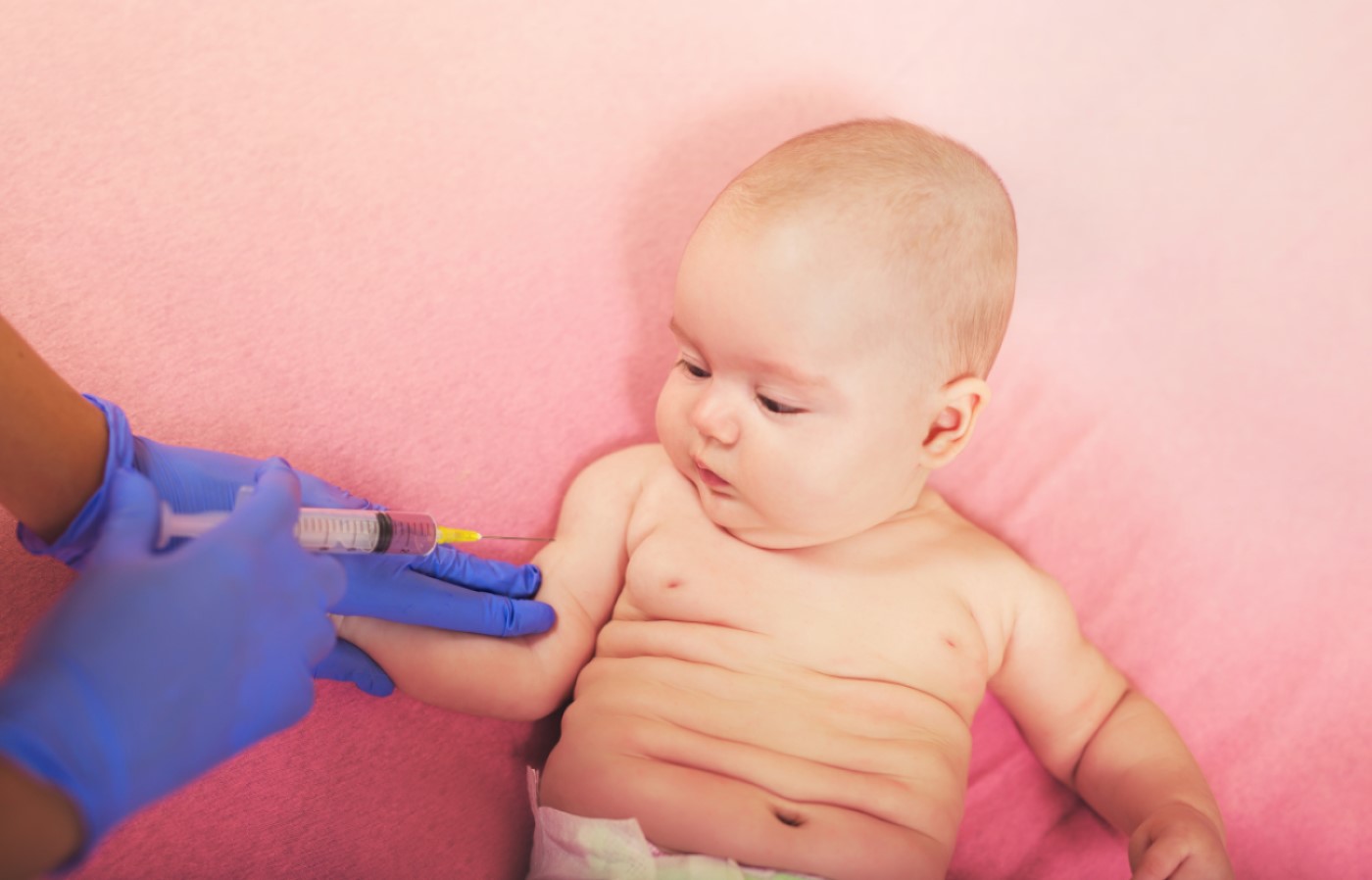
Vaccine that combined diphtheria, tetanus, acellular pertussis, inactivated polio and hepatitis B antigens was licensed
On Dec. 13, 2002, the U.S. Food and Drug Administration (FDA) announced it had licensed a combined diphtheria and tetanus toxoids and acellular pertussis adsorbed (DTaP), hepatitis B (HepB) (recombinant) and inactivated poliovirus vaccine (IPV), DTaP-HepB-IPV (PEDIARIX™, SmithKline Beecham Biologicals, Rixensart, Belgium) for use in infants ages 2, 4, and 6 months.
Except for fever, the rates of most solicited local and systemic adverse events following DTaP-HepB-IPV were comparable to rates observed following separately administered U.S.-licensed vaccines.
Tags:
Source: U.S. Centers for Disease Control and Prevention
Credit:
