The first pneumococcal vaccine was licensed
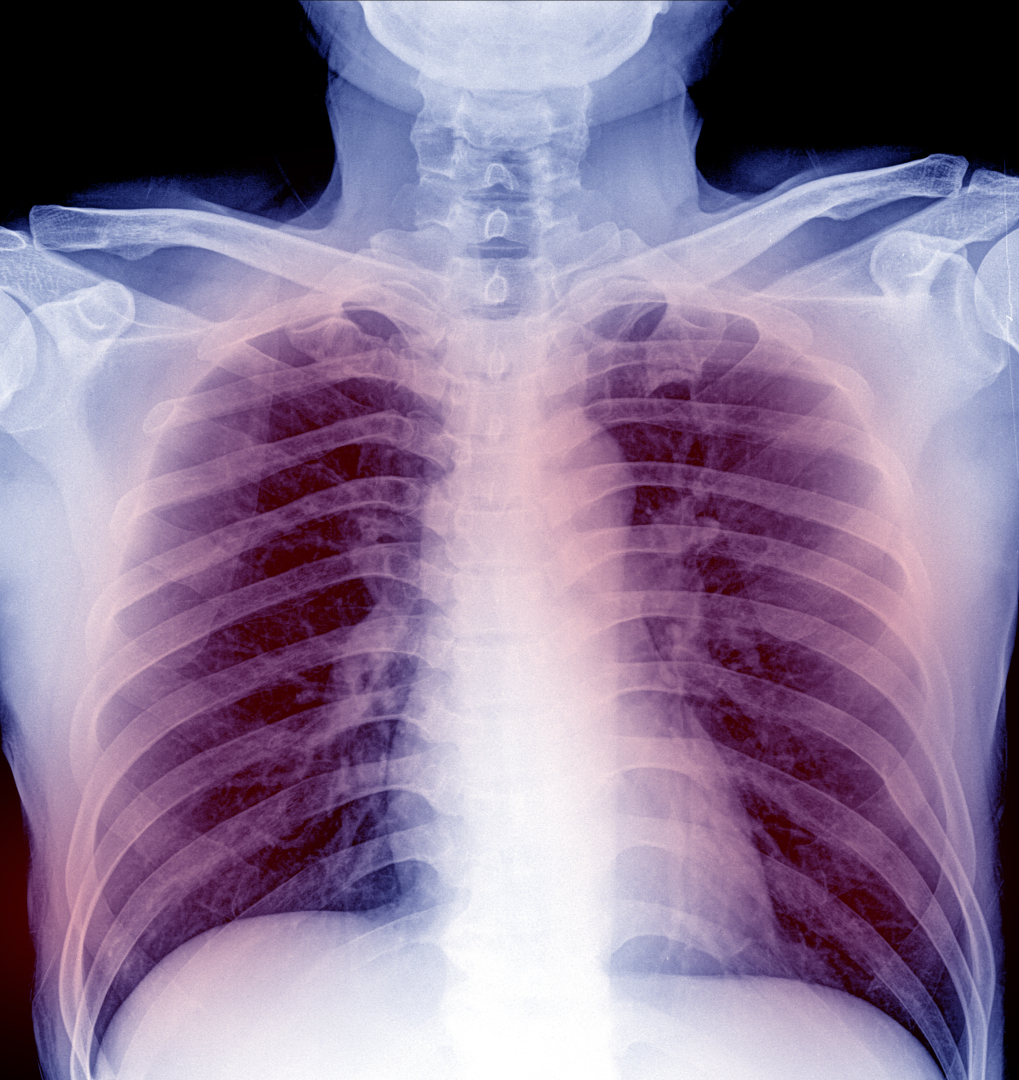
On Nov. 21, 1977, the U.S. Food and Drug Administration (FDA) licensed the first pneumococcal vaccine containing 14 serotypes (of the 83 known serological groups) that comprised 80% of all bacteremic pneumococcal infections in the U.S. A 23-valent pneumococcal polysaccharide followed in 1983.
The vaccine, developed by Merck Sharp & Dohme in West Point, Pa., offered protection against pneumococcal disease, a name for any infection caused by bacteria called Streptococcus pneumoniae, or pneumococcus. People spread pneumococcal bacteria to others through direct contact with respiratory secretions, like saliva or mucus.
In the era before antibiotics, S. pneumoniae was estimated to be the cause of 95% of all cases of pneumonia. Currently, however, S. pneumoniae accounts for up to 15% of pneumonia cases in the United States and 27% of cases worldwide today. Blood cultures are positive in only 20% to 25% of all pneumonia cases that are caused by S. pneumonia making it a challenging diagnosis for the clinician.
Tags:
Source: National Library of Medicine
Credit:
