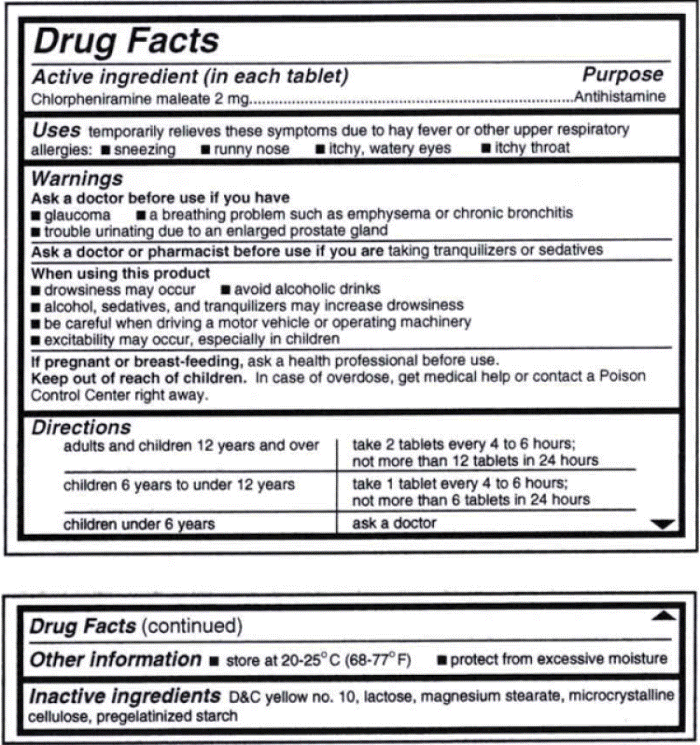
The FDA mandated OTC drugs labels contain information in a standardized, consumer-friendly format
On Mar. 17, 1999, the Food and Drug Administration (FDA) published a regulation mandating that labels of all over-the-counter drugs contain information in a standardized, consumer-friendly format.
These “Drug Facts” labels, similar in format to the Nutrition Facts label for foods, are designed to provide easy-to-find information to help patients use the product safely and effectively.
Tags:
Source: U.S. Food and Drug Administration
Credit:
