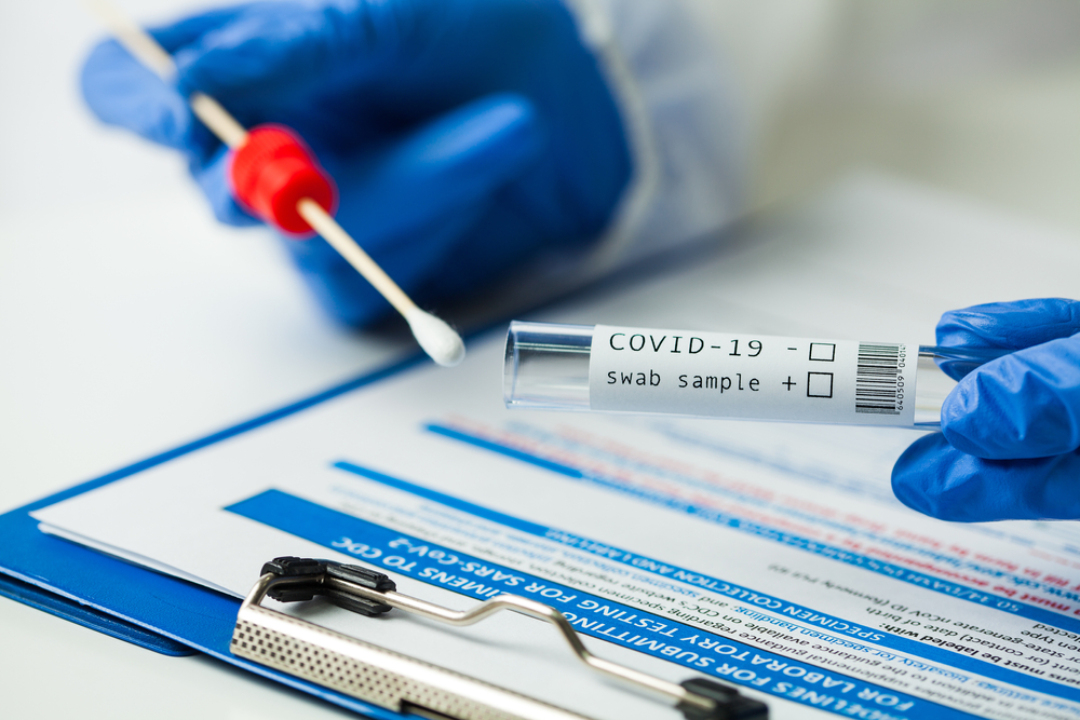
The FDA issued EUA for first antigen test to help in rapid detection of virus that causes COVID-19 in patients
On May 8, 2020, the U.S. Food and Drug Administration (FDA) issued the first emergency use authorization (EUA) for a COVID-19 antigen test, a new category of tests for use in the ongoing pandemic.
These diagnostic tests quickly detect fragments of proteins found on or within the virus by testing samples collected from the nasal cavity using swabs.
The EUA was issued to Quidel Corp. for the Sofia 2 SARS Antigen FIA. This test is authorized for use in high and moderate complexity laboratories certified by Clinical Laboratory Improvement Amendments (CLIA), as well as for point-of-care testing by facilities operating under a CLIA Certificate of Waiver.
Tags:
Source: U.S. Food and Drug Administration
Credit:
