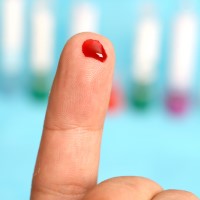
The FDA issued an EUA to Symbiotica for the COVID-19 self-collected antibody test system
On Apr. 5, 2021, the FDA issued an Emergency Use Authorization (EUA) to Symbiotica for the COVID-19 Self-Collected Antibody Test System, making it the first serology test authorized for use with a blood sample self-collected at-home.
The Symbiotica COVID-19 Self-Collected Antibody Test System requires a prescription from a health care provider. Samples collected at home are sent to a Symbiotica laboratory for analysis.
Tags:
Source: U.S. Food and Drug Administration
Credit:
