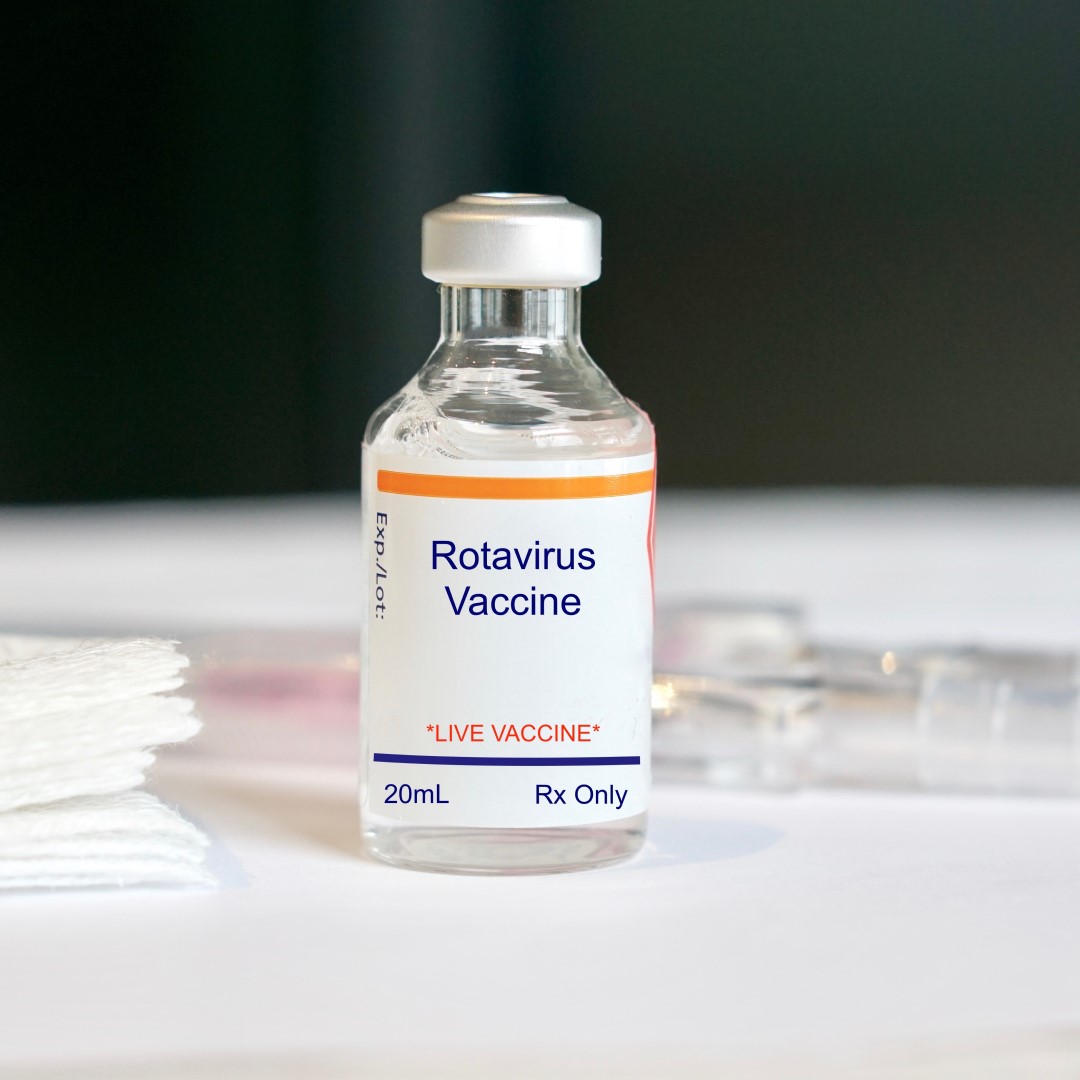
The CDC’s advisory Committee recommended routine immunizations for children and adolescents of rotavirus and HPV
On Jan. 6, 2006, the U.S. Centers for Disease Control and Prevention’s (CDC) advisory Committee on Immunization Practices (ACIP) recommended a childhood and adolescent immunization schedule to ensure that the schedule was current with changes in vaccine formulations and reflects revised recommendations for the use of licensed vaccines, including those newly licensed.
Hepatitis A vaccine was universally recommended for all children at age 1 year (12–23 months). The 2 doses in the series should be administered at least 6 months apart.
Tags:
Source: U.S. Centers for Disease Control and Prevention
Credit:
