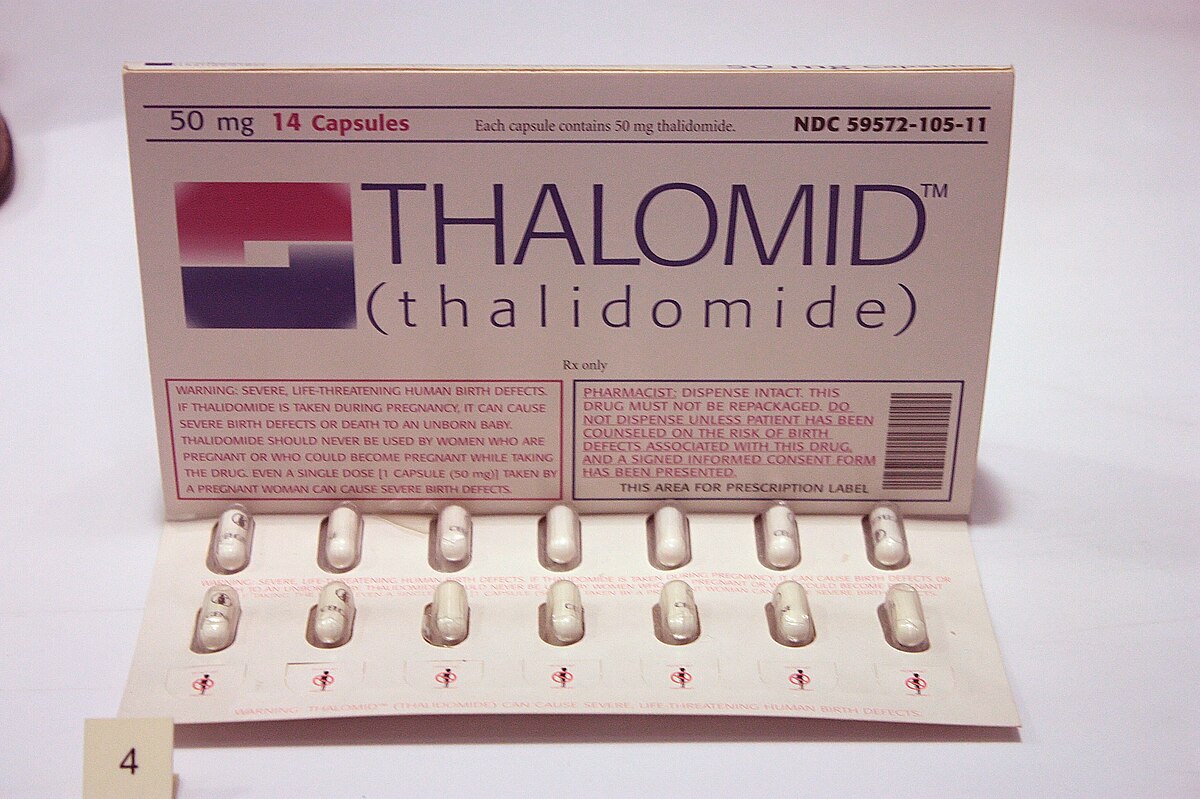
Thalidomide-caused birth defects lead to strengthened drug regulation
On Jul. 15, 1962, Thalidomide, a new sleeping pill developed by the German company Grunenthal, was found to have caused birth defects in thousands of babies born in western Europe. News reports on the role of U.S. Food and Drug Administration (FDA) medical officer Dr. Frances O. Kelsey in keeping the drug off the American market aroused public support for stronger drug regulation.
This tragedy gave new impetus to a bill intended to enhance drug regulation, the Kefauver-Harris amendments to the Federal Food, Drug, and Cosmetic Act, which became law Oct. 10, 1962.
However, the Kefauver-Harris amendments did not require premarket approval of drugs brought to the market before the passage of the FD&C Act in 1938.
Tags:
Source: U.S. Food and Drug Administration
Credit:
