KPWHRI announced volunteers needed for COVID-19 vaccine-booster trial
On Jun. 16, 2021, Kaiser Permanente Washington Health Research Institute (KPWHRI) announced that was recruiting volunteers in the…

On Jun. 16, 2021, Kaiser Permanente Washington Health Research Institute (KPWHRI) announced that was recruiting volunteers in the…
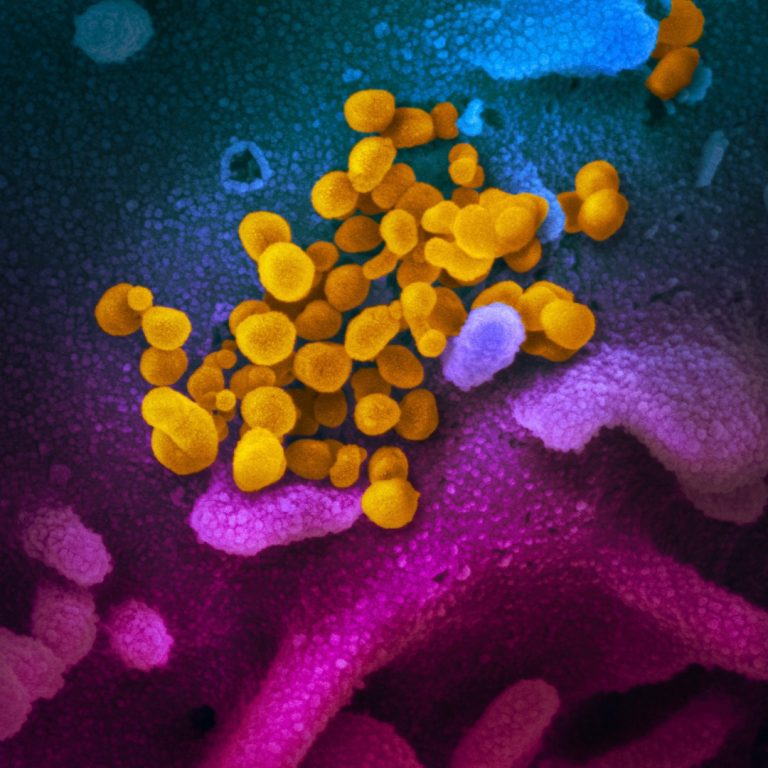
On Jun. 14, 2021, Cocrystal Pharma announced the selection of CDI-45205 as the lead compound for further development…

On Jun. 1, 2021, a study by researchers at the National Institutes of Health and their colleagues found…

On May 28, 2021, the Oregon Department of Agriculture (ODA) lifted the quarantine on the Oregon mink farm…

On May 27, 2021, Innovation Pharmaceuticals announced that patient enrollment in the Companyメs 120-patient, Phase 2 clinical trial…
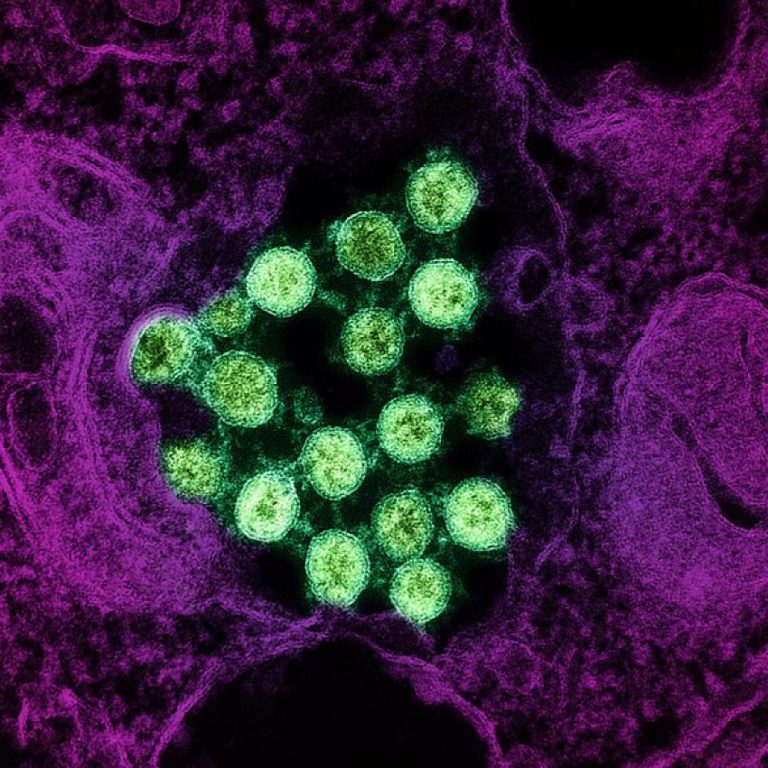
On May 27, 2021, Seattle Children’s researchers published a study that uncovered a deeper understanding of why people…

On May 26, 2021, Altimmune announced new results from a preclinical study demonstrating the ability of its AdCOVID…

On May 24, 2021, the World Health Organization (WHO) and the Swiss Confederation signed a Memorandum of Understanding…

On May 24, 2021, LabCorp reported that nearly 87% of naturally infected COVID-19 patients maintained antibodies to SARS-CoV-2…

On May 21, 2021, world leaders the Global Health Summit adopted an agenda to overcome the COVID-19 pandemic,…

On May 7, 2021, GlaxoSmithKline and Vir Biotechnology announced that the European Medicines Agency (EMA) has started a…

On May 5, 2021, Hoth Therapeutics announced it has expanded its sponsored research agreement with Virginia Commonwealth University…
On May 3, 2021, the World Health Organization (WHO) announced the end of the 12th outbreak of Ebola…

On May 3, 2021, Vaxart announced that new data obtained from its Phase I COVID-19 trial added to…

On Apr. 28, 2021, VBI Vaccines announced that preclinical data of VBIメs enveloped virus-like particle (eVLP) vaccine candidate,…

On Apr. 28, 2021, Innovation Pharma announced that enrollment had surpassed 50 percent of the total targeted number…

On Apr. 15, 2021, the National Institutes of Health announced that the experimental antiviral drug MK-4482 significantly decreased…

On Apr. 13, 2021, a clinical trial testing the safety and efficacy of an investigational monoclonal antibody for…

On Apr. 7, 2021, the Centers for Disease Control and Prevention (CDC) announced it had awarded $3 billion…

On Apr. 5, 2021, Novavax announced the initiation of crossover arms in two ongoing clinical trials of NVX-CoV2373,…

On Apr. 5, 2021, Anixa Biosciences announced that based on Proof of Concept animal study results, it was…
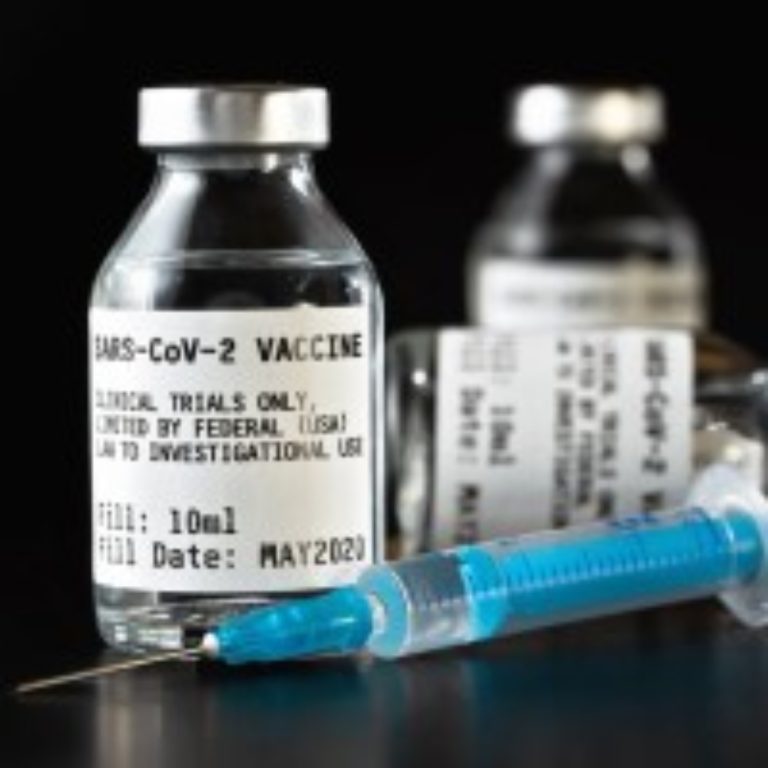
On Apr. 1, 2021, Kaiser Permanente Washington Health Research Institute (KPWHRI) announced that an investigational vaccine designed to…

On Apr. 1, 2021, Arbutus Biopharma, X-Chem and Proteros Biostructures announced hat they had entered into a discovery…
On Apr. 1, 2021, Kaiser Permanente Washington Health Research Institute (KPWHRI) announced that an investigational vaccine designed to…

On Mar. 30, 2021, Qx Therapeutics announced that the FDA had cleared the companyメs Investigational New Drug application…

On Mar. 29, 2021, an international research team led by Oxford University scientists announced they had developed a…
On Mar. 25, 2021, an international team of scientists announced they had found evidence that SARS-CoV-2 infects cells…
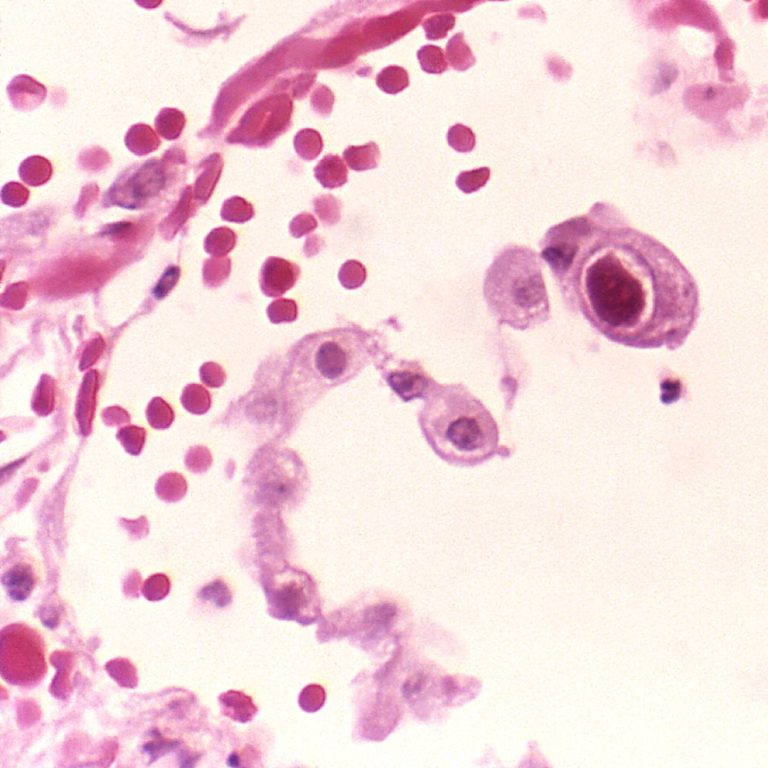
On Mar. 25, 2021, about two decades after first devising a new kind of vaccine, Oregon Health &…

On Mar. 24, 2021, Virginia Tech researchers announced that their candidate Coronavirus vaccines, prevented pigs from becoming ill…

On Mar. 23, 2021, Pfizer announced that it was progressing to multiple ascending doses after completing the dosing…