Medigen Vaccines obtained TFDA phase 2 IND approval for COVID19 vaccine
On Dec. 30, 2020, Medigen Vaccine Biologics announced that it had obtained TFDA Phase 2 IND approval of…

On Dec. 30, 2020, Medigen Vaccine Biologics announced that it had obtained TFDA Phase 2 IND approval of…

On Nov. 24, 2020, MediciNova announced development progress on its intranasal SARS-CoV-2 vaccine for COVID-19 utilizing BC-PIV, a…

On Sept. 11, 2020, MediciNova announced development progress on its intranasal SARS-CoV-2 vaccine for COVID-19 utilizing BC-PIV, a…

On Sept. 9, 2020, iBio announced that it had selected IBIO-201, its LicKMル-ubunit vaccine, as its leading candidate…
On Sept. 1, 2020, MediciNova announced development progress on its intranasal SARS-CoV-2 vaccine for COVID-19 utilizing BC-PIV, a…

On Jan. 31, 2018, the FDA published the first Red Book, the successor to 1949 “black book,” officially…

On Dec. 20, 2011, the U.S. Food and Drug Administration (FDA) announced approval of the first and only…

In 1982, the U.S. Food and Drug Administration issued a publication, known as the Redbook, that described the…
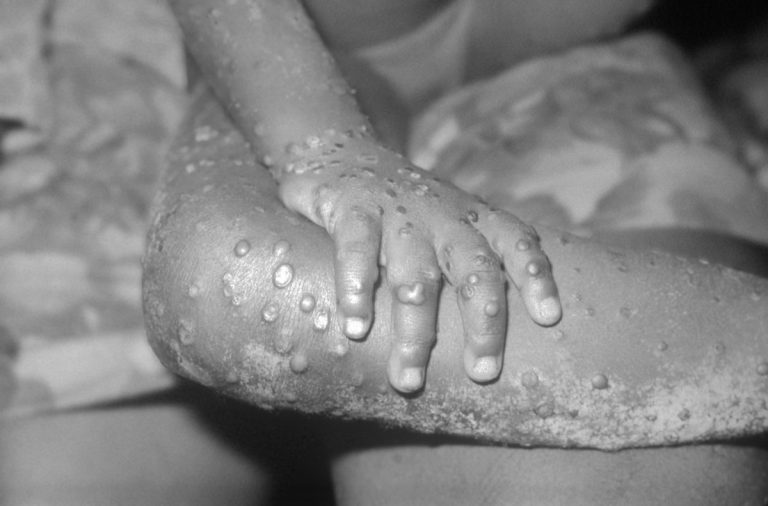
In 1970, the first human case of mpox was reported in the Democratic Republic of the Congo (DRC)…

In 1958, mpox (MPVX) was first discovered when two nonfatal outbreaks of a pox-like disease were reported in…

In 1955, The National Cancer Chemotherapy Program was initiated. It was administered and integrated by the Division of…

In 1949, U.S. Food and Drug Administration (FDA) published guidance to industry for the first time. This guidance,…