FDA approved Roche’s Actemra for treatment of COVID-19 in hospitalised adults
On Dec. 21, 2022, Roche announced that the U.S. Food and Drug Administration (FDA) had approved Actemraᆴ (tocilizumab)…

On Dec. 21, 2022, Roche announced that the U.S. Food and Drug Administration (FDA) had approved Actemraᆴ (tocilizumab)…

On Feb. 11, 2022, Roche announced that Actemraᆴ/RoActemraᆴ (tocilizumab) intravenous (IV) had been granted World Health Organization (WHO)…

On Dec. 6, 2021, Roche announced that the European Medicines Agencyメs Committee for Medicinal Products for Human Use…
On Jul. 6, 2021, the World Health Organization announced updated patient care guidelines to include interleukin-6 receptor blockers,…

On Jun. 14, 2021 Humanigen announced it had initiated a rolling review submission for Marketing Authorization (MA) by…

On Mar. 29, 2021, Humanigen announced positive topline results from its Phase 3 clinical trial evaluating the efficacy…

On Jul. 22, 2020, a new study led by Albert Einstein College of Medicine and Montefiore Health System…
In 2004, the U.S. Congress passed the Anabolic Steroid Control Act which enacted a ban on over-the-counter steroid…
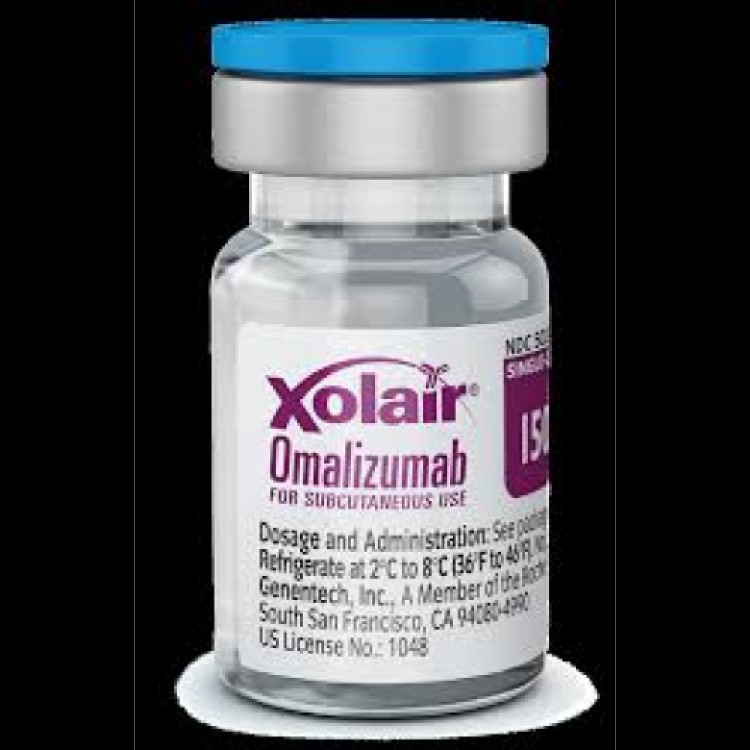
On Jun. 20, 2003, Genentech drug Xolair (omalizumab) was approved by the U. S. Food and Drug Administration…

In 1990, responding to increasing illicit traffic, the U.S. Congress passed the Anabolic Steroid Act, which identified anabolic…

On Oct. 27, 1986, President Ronald Reagan signed the the Anti-Drug Abuse Act of 1986. The Act changed…