US measles cases soar to 588 so far this year as South Carolina confirms 58 new infections
On Jan. 30, 2026, the U.S. Centers for Disease Control and Prevention (CDC) confirmed 172 new measles cases…

On Jan. 30, 2026, the U.S. Centers for Disease Control and Prevention (CDC) confirmed 172 new measles cases…

On Jan. 27, 2026, South Carolina reported a surge to 789 measles cases, state health data showed, overtaking…

On Jan. 15, 2026, the USA is experiencing a resurgence of measles, despite the widespread availability of the…

On Jan. 14, 2026, a Stanford University–led research team estimates that county-level US nonmedical exemptions to childhood vaccine…

On Jan. 2, 2026, U.S. states will no longer be required to report how many children they vaccinate…

On Dec. 29, 2025, measles cases nationwide have reached 2,012, the Centers for Disease Control and Prevention (CDC)…

On Sept. 19, 2025, the state health officials of the members of the West Coast Health Alliance, including…

On Aug. 18, 2025, the World Health Organization announced Nepal has eliminated rubella as a public health problem,…
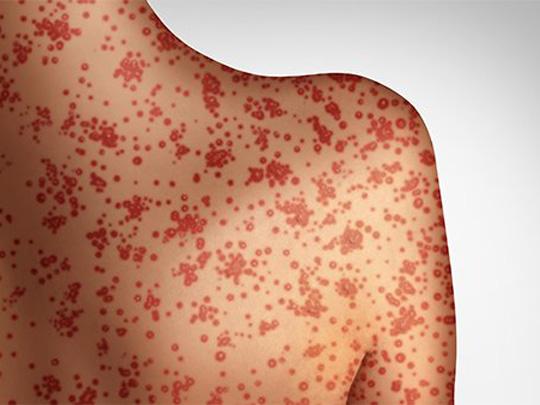
On Jul. 10, 2025, the AP reported that he U.S. is having its worst year for measles spread…

On Jun. 9, 2025, Health and Human Services Secretary Robert F. Kennedy Jr. has fired all 17 members…

On Nov. 21, 2024, the U.S. Centers for Disease Control and Prevention (CDC) reported that by the week…

On Nov. 14, 2024, the World Health Organization (WHO) and the U.S. Centers for Disease Control and Prevention…

On Aug. 28, 2024, survey data from the Annenberg Public Policy Center (APPC) found that the number of…

On Mar. 6, 2023, Merck announced that the U.S. Food and Drug Administration had approved the addition of…

On Aug. 14, 2020, in the U.S., despite high rates of coverage (>90%) at the national level for…
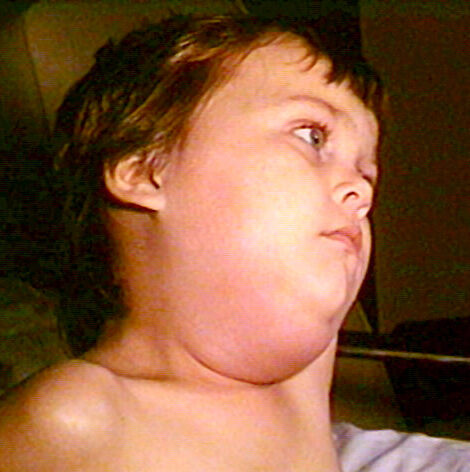
On Jan. 12, 2018, the U.S. Centers for Disease Control and Prevention’s Immunization Practices Advisory Committee Advisory Committee on…
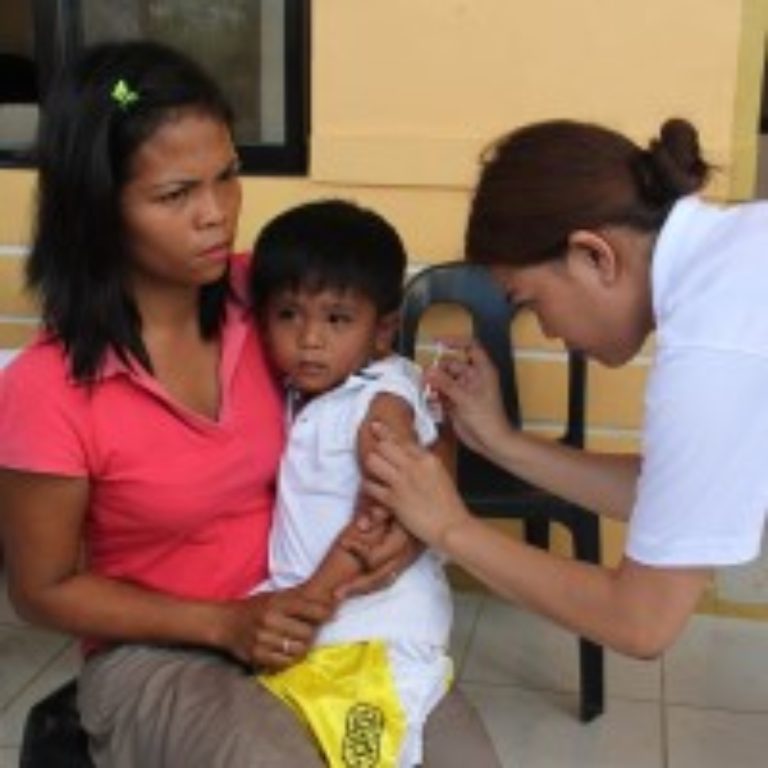
On Sept. 27, 2016, the Region of the Americas was the first in the world to have eliminated…
On Apr. 29, 2015, the Pan American Health Organization ᅠdeclared that the Americas region has become the first…

On Jun. 14, 2013, the U.S. Centers for Disease Control and Prevention’s (CDC) Advisory Committee on Immunization Practices…

On Oct. 21, 2009, Merck announced that the company will not resume production of monovalent measles, mumps, and…
On Feb. 12, 2009, the Vaccine Court ruled that MMR vaccine, when administered with thimerosal-containing vaccines, does not…

On Mar. 14, 2008, the U.S. Centers for Disease Control and Prevention (CDC) updated its recommendations for administering…

On Sept. 6, 2005, the vaccine that combined the measles, mumps, rubella, and varicella antigens (Proquad by Merck)…

On Mar. 21, 2005, the U.S. Centers for Disease Control and Prevention (CDC) announced that a major public…
In 2004, Rubella, also known as German measles, was declared eliminated in the U.S. Before the rubella vaccination…
On Jan. 11, 1991, recommendations of Immunization Practices Advisory Committee (ACIP) for routine Hib vaccination for infants beginning…

On Apr. 13, 1990, the Immunization Practices Advisory Committee (ACIP) recommendations for use of any of the three…
On Jan. 22, 1988, the Centers for Disease Control and Prevention’s (CDC) Immunization Practices Advisory Committee (ACIP) recommended…

In 1986, the National Childhood Vaccine Injury Act was enacted by Congress. The Department of Health and Human…

In 1979, the U.S. Food and Drug Administration (FDA) licensed the RA 27/3 (human diploid fibroblast) strain of…