Global diarrheal deaths drop, but children and elderly remain most at risk
On Dec. 18, 2024, the Institute for Health Metrics and Evaluation reported that diarrhoeal diseases claim more than…
On Dec. 18, 2024, the Institute for Health Metrics and Evaluation reported that diarrhoeal diseases claim more than…

On Oct. 21, 2011, the Food and Drug Administration (FDA) announced it had approved revised prescribing information and…

On Mar. 23, 2010, the U.S. Food and Drug Administration (FDA) recommended that physicians temporarily suspend use of…

On Apr. 3, 2008, the U.S. Food and Drug Administration (FDA) approved new rotavirus vaccine (Rotarix) for use…

On Feb. 3, 2006, Rotavirus vaccine, live, oral, pentavalent (RotaTeq by Merck) was licensed for use in infants…

On May 4, 2004, the National Institute of Allergy and Infectious Diseases (NIAID), awarded a new license agreement…

On Oct. 22, 1999, the Advisory Committee on Immunization Practices (ACIP), after a review of scientific data from…
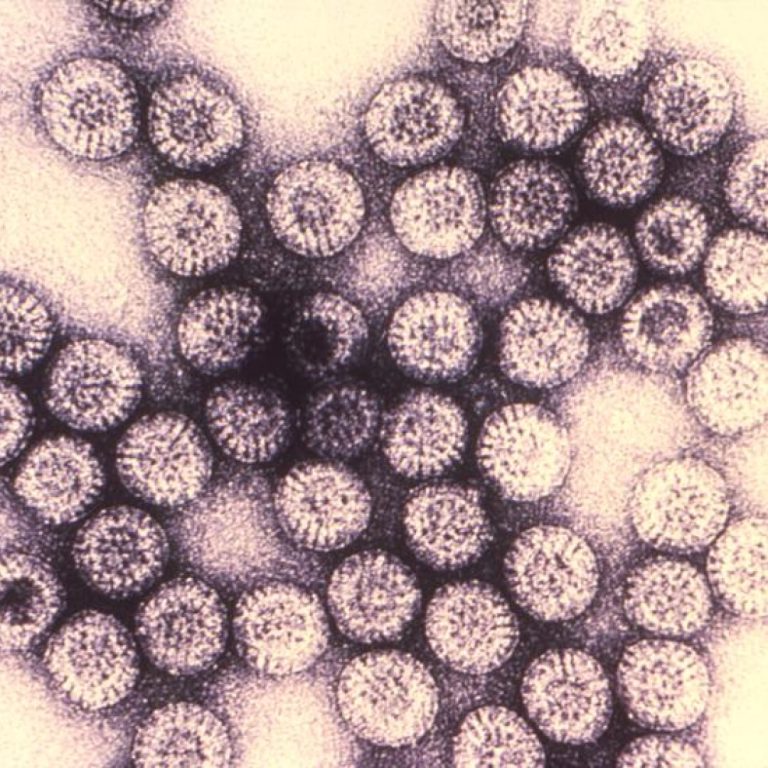
On Oct. 15, 1999, Wyeth-Lederle Vaccines announced that it was withdrawing the Rotashield vaccine from the market and…

On Jan. 15. 1999, the U.S. Centers for Disease Control and Prevention’s (CDC) Advisory Committee on Immunization Practices…

On Aug. 31, 1998, Rotashield (Wyeth Laboratories, Marietta, Pennsylvania) was licensed by the U.S. Food and Drug Administration…

On Aug. 26, 1998, Bill and Melinda Gates announced the establishment of the Bill and Melinda Gates Children’s Vaccine…

In 1990, the U.S. Centers for Disease Control and Prevention (CDC) reported viral causes of gastroenteritis, helping identify…

In 1972, The Wistar Institute was designated the first National Cancer Institute (NCI) Cancer Center in basic research…