The Jackson Laboratory to provide expanded Coronavirus testing for Maine hospitals
On Apr. 14, 2020, the Jackson Laboratory (JAX) in collaboration with several Maine regional hospitals, announced additional coronavirus…

On Apr. 14, 2020, the Jackson Laboratory (JAX) in collaboration with several Maine regional hospitals, announced additional coronavirus…

On Apr. 13, 2020, Blackstone and Alnylam Pharma announced a broad strategic collaboration under which Blackstone will provide…

On Apr. 9, 2020, FUJIFILM announced initiation of a U.S. phase II clinical trial to evaluate the safety…

On Apr. 9, 2020, Pfizer and BioNTech disclosed additional details of their collaboration to advance candidates from BioNTech’s…

On Apr. 6, 2020, Emory’s Vaccine and Treatment Evaluation Unit (VTEU) announced its participation in a clinical trial…

On Apr. 6, 2020, VGX announced successful implementation of new RNA production services through completion of all Phase…

On Apr. 2, 2020, Vir Biotech and Alnylam Pharma announced an expansion to their broad multi-target existing collaboration…

On Mar. 31, 2020, FUJIFILM announced initiation of a phase III clinical trial to evaluate the safety and…

On Mar. 24, 2020, MatMaCorp announced the successful evaluation of a genetic test to detect African swine fever…

On Mar. 17, 2020, Pfizer and BioNTech SE announced the companies have agreed to a letter of intent…

On Mar. 16, 2020, Moderna announced that the first participant had been dosed in the Phase 1 study…
On Mar. 15, 2020, Fosun Pharma and BioNTech announced a strategic development and commercialization collaboration to advance BioNTech’s…

On Mar. 5, 2020, Quest Diagnostics announced it will launch a coronavirus (COVID-19) test service. The new test…

On Mar. 4, 2020, Vir Biotech and Alnylam Pharmaceuticals announced an expansion of their existing collaboration to include…
On Mar. 3, 2020, Moderna announced that enrollment was complete for all three dose cohorts of the Phase…

On Feb. 24, 2020, Moderna announced it had released the first batch of mRNA-1273, the Company’s vaccine against…

On Jan. 30, 2020, the University of Washington (UW) announced that a new center was being created with…

On Jan. 23, 2020, Moderna announced a new collaboration to develop an mRNA vaccine against the novel coronavirus…

On Oct. 1, 2019, the National Institutes of Health (NIH) awarded a $3.5 million grant to Georgia Institute…
On Aug. 20, 2019, the eleventh U.S. CRISPR-Cas9 patent was awarded to University of California (UC), the University…

On Sept. 11, 2018, Joan Steitz, Sterling Professor of Molecular Biophysics and Biochemistry at Yale, received the 2018ᅠ…

On Aug. 22, 2018, University of California, San Diego (UCSD) researchers with colleagues in Spain and Finland, described…

On Aug. 6, 2013, Pacific Northwest Diabetes Research Institute (PNDRI) received a $2 million National Institutes of Health…
On Oct. 20, 2011, researchers announced they had sequenced genomic DNA and RNA from the marijuana strain Purple…
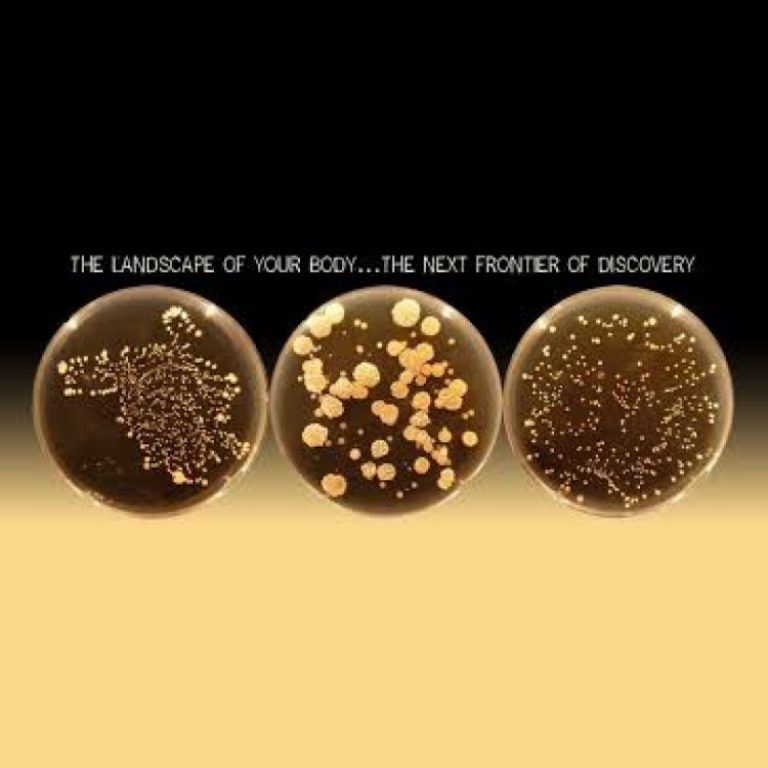
On Jan. 3, 2011, the Belly Button Biodiversity project was launched to investigate the microbes inhabiting our navels…

On Jul. 22, 2010, MDRNA announced that MDRNA shareholders approved the issuance of shares of MDRNA common stock…
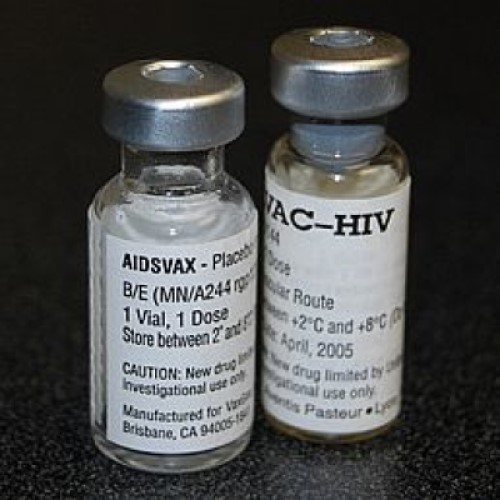
On Sept. 24, 2009, the second Phase III HIV-1 vaccine trial, also known as RV144, that began in…

On Feb. 25, 2008, Oregon State University’s James Carrington, a professor of botany and plant pathology and a…

In 2008, the U.S. Centers for Disease Control and Prevention (CDC) received U.S. Food and Drug Administration (FDA)…

On Sept. 18, 2006, Integrated DNA Technologies (IDT) announced the acquisition of RNA-TEC, based in Leuven, Belgium. The…