University of Missouri launched rabies lab for pets traveling abroad
On Jun. 6, 2023, the University of Missouri College of Veterinary Medicine announced it had launched a rabies…

On Jun. 6, 2023, the University of Missouri College of Veterinary Medicine announced it had launched a rabies…

On May 20, 2020, Bharat Biotech and Thomas Jefferson University of Philadelphia announced an exclusive deal to develop…
On May 16, 2018, the U.S. Centers for Disease Control and Prevention (CDC) released information about a new…

On Mar. 19, 2010, the Immunization Practices Advisory Committee (ACIP) recommended use of a reduced (4-dose) vaccine schedule…

On Oct. 20, 1997, the Food and Drug Administration (FDA) licensed Purified Chick Embryo Cell (PCEC, RabAvert®) vaccine…

On Jun. 9, 1980, the rabies human diploid-cell vaccine (Imovax Rabies by Merieux, now Sanofi, and Wyvac by…
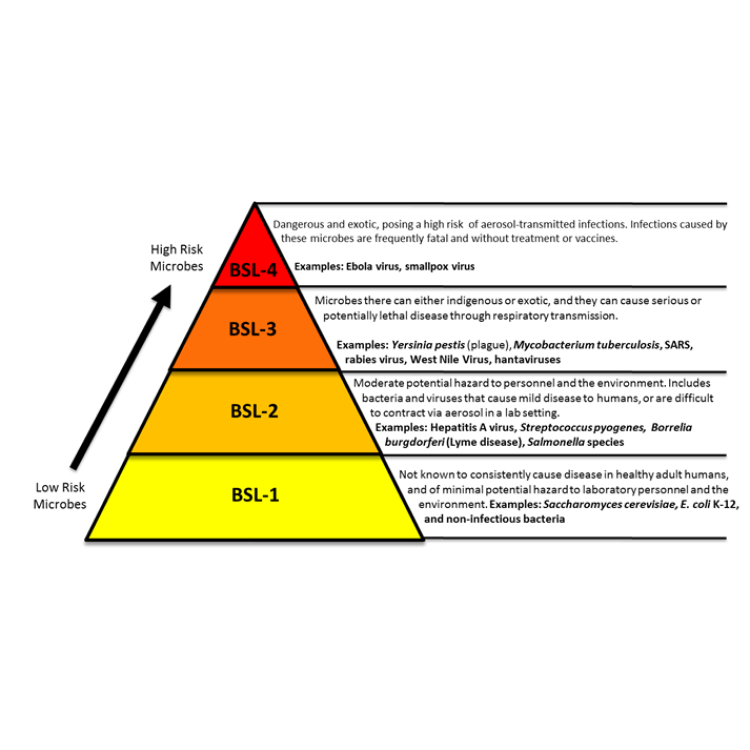
In 1978, the U.S. Centers for Disease Control and Prevention (CDC) completed construction of a new hot lab…

In 1972, The Wistar Institute was designated the first National Cancer Institute (NCI) Cancer Center in basic research…

In 1959, the Centers for Disease Control and Prevention (CDC) developed a fluorescent antibody test for rabies, with…

In 1953, the U.S. Centers for Disease Control and Prevention (CDC) reported the first transmission of rabies by…

In 1945, Karl Habel cultivated mumps virus in embryonated eggs and devised serological tests for its presence. Habel…
On Sept. 6, 1940, Karl Habel produced an improved, killed rabies vaccine that eliminated foreign brain tissue that…

On Oct. 11, 1938, the Squibb Biological Laboratories, New Brunswick, New Jersey, established a new laboratory for the…

In 1914, rabies vaccine was first licensed in the U.S. The H. K. Mulford Company, founded in Philadelphia…

In 1908, Arthur Marston Stimson developed a better method for rabies vaccine preparation so it could be sent…

In 1895, the H. K. Mulford Company, founded in Philadelphia, became the first commercial producer of diphtheria antitoxin…

On Nov. 14, 1888, following Louis Pasteur’s successful international appeal for funds, the Pasteur Institute opened it doors…

On Jul. 6, 1885, Louis Pasteur’s anti-rabies vaccine was successfully tested on nine-year old Joseph Meister who had…

In 1884, the first live attenuated viral vaccine (rabies) was developed by Louis Pasteur, using dessicated brain tissue…

In 1877, Louis Pasteur noted that some bacteria die when cultured with certain other bacteria, indicating that some…