COVID-19 coronavirus spike holds infectivity details
On Feb. 24, 2020, researchers reported that the spikes crowning the new coronavirus that causes COVID-19 atypical pneumonia…
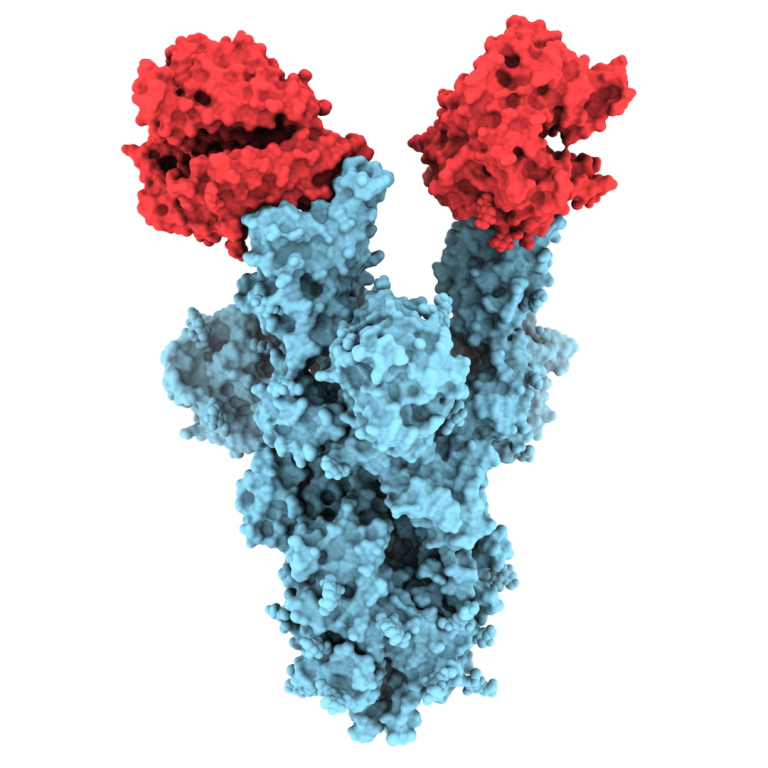
On Feb. 24, 2020, researchers reported that the spikes crowning the new coronavirus that causes COVID-19 atypical pneumonia…
On Jan. 23, 2020, the new cluster of viral pneumonia cases originating in Wuhan, China, marked the third…
On Jan. 9, 2020, the World Health Organization announced that a novel coronavirus had been identified as the…

On Dec. 31, 2019, the World Health Organization (WHO) declared the Coronavirus outbreak a global pandemic. To date,…
On Dec. 19, 2019, the WHO announced that PNEUMOSIL, a vaccine against a leading cause of deadly childhood…
On Nov. 22, 2019, the Centers for Disease Control and Prevention”s (CDC) Advisory Committee on Immunization Practices (ACIP)…
On Sept. 27, 2016, the Region of the Americas was the first in the world to have eliminated…

On Jul. 12, 2016, Pfizer announced that Prevnar 13ᆴ (Pneumococcal 13-valent Conjugate Vaccine [Diphtheria CRM197 Protein]) received FDA…

On Sept. 4, 2015, the U.S. Centers for Disease Control and Prevention’s (CDC) Advisory Committee on Immunization Practices…

On Sept. 19, 2014, the Centers for Disease Control and Prevention”s (CDC) Advisory Committee on Immunization Practices (ACIP)…

On Jun. 28, 2013, the Advisory Committee on Immunization Practices (ACIP) recommended routine use of 13-valent pneumococcal conjugate…

On Jan. 25, 2013, Pfizer announced the FDA had granted approval for the expansion of the companyメs pneumococcal…
On Dec. 30, 2011, Pfizer announced that the U.S. Food and Drug Administration (FDA) had granted approval of…

On Feb. 24, 2010, a 13-valent pneumococcal conjugate vaccine (PCV13 [Prevnar 13, Wyeth Pharmaceuticals, a subsidiary of Pfizer])…

On Nov. 16, 2009, the U.S. Centers for Disease Control and Prevention (CDC) recommended a single dose of…

On Nov. 12, 2009, the Global Coalition against Child Pneumonia was established to raise awareness about the toll…

On Oct. 7, 2005, a new Federal Medicare rule became effective that required all long-term care facilities to…
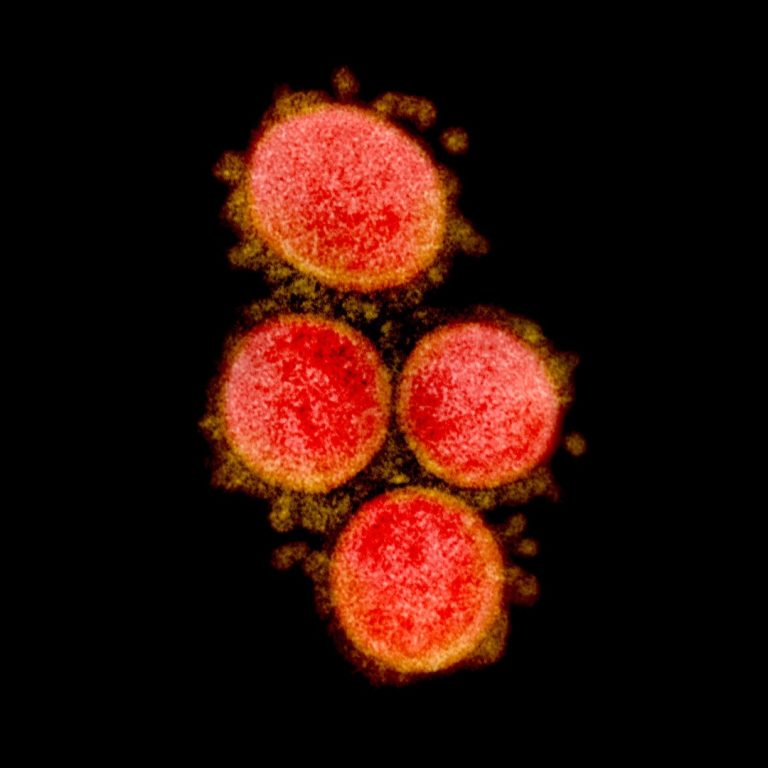
On Mar. 6, 2003, the Singapore Ministry of Health (MOH) and the World Health Organization (WHO), announced they…

On April 4, 1997, the Immunization Practices Advisory Committee (ACIP) recommended that the vaccine be used more extensively…
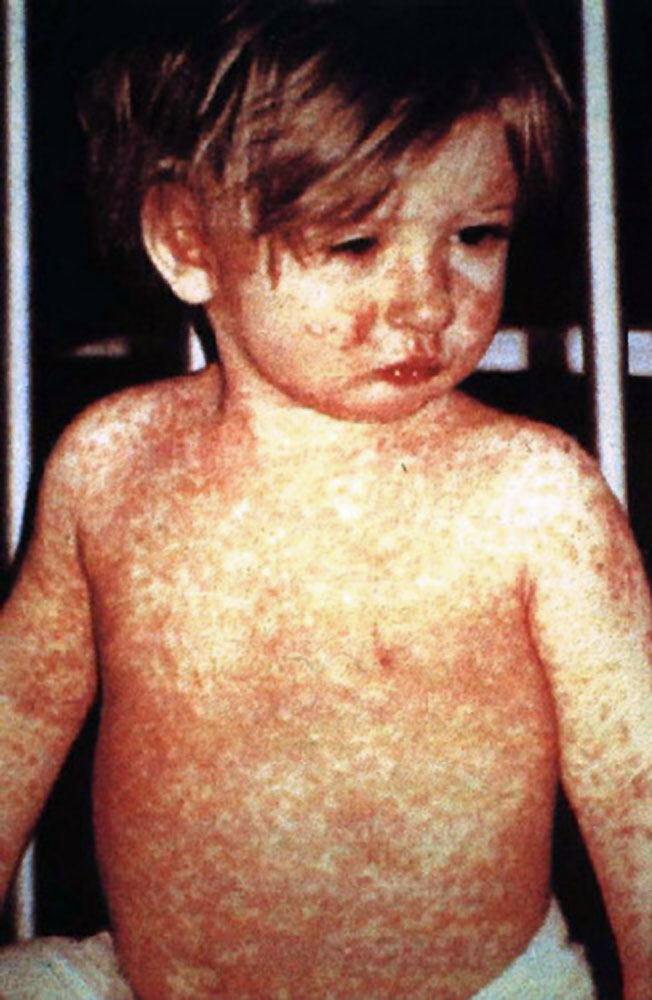
In 1989, recommendations for routine 2nd doses of measles-containing vaccine were issued by both ACIP and the American…

In 1986, Dr. Marilyn Hughes Gaston while deputy branch chief of the Sickle Cell Disease Branch at the…
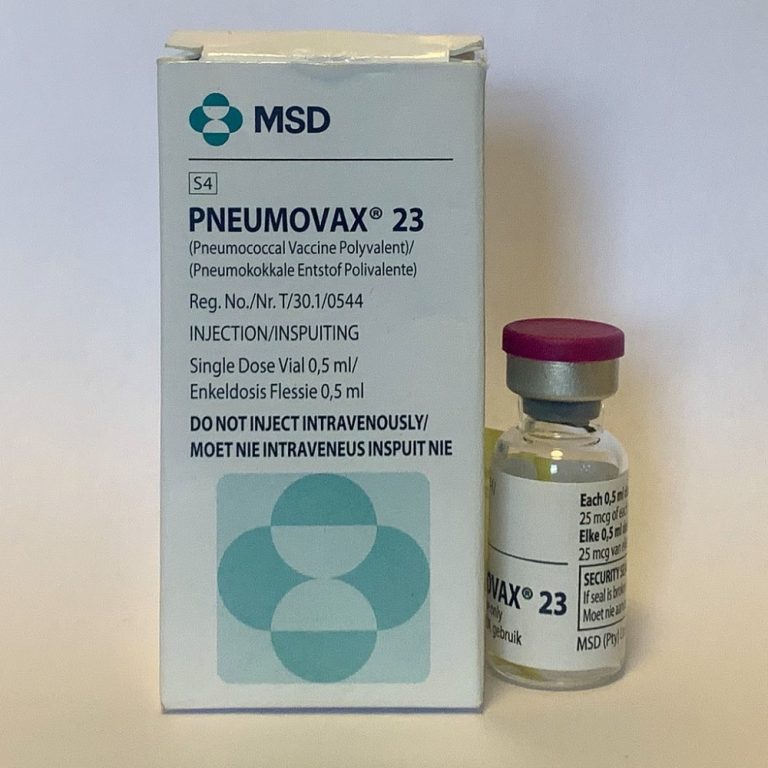
On Jun. 30, 1983, the U.S. Food and Drug Administration (FDA) licensed two enhanced pneumococcal polysaccharide vaccines (Pneumovax…

On Jul. 1, 1981, as a result of the enactment of Public Law 96-611 passed by the U.S….
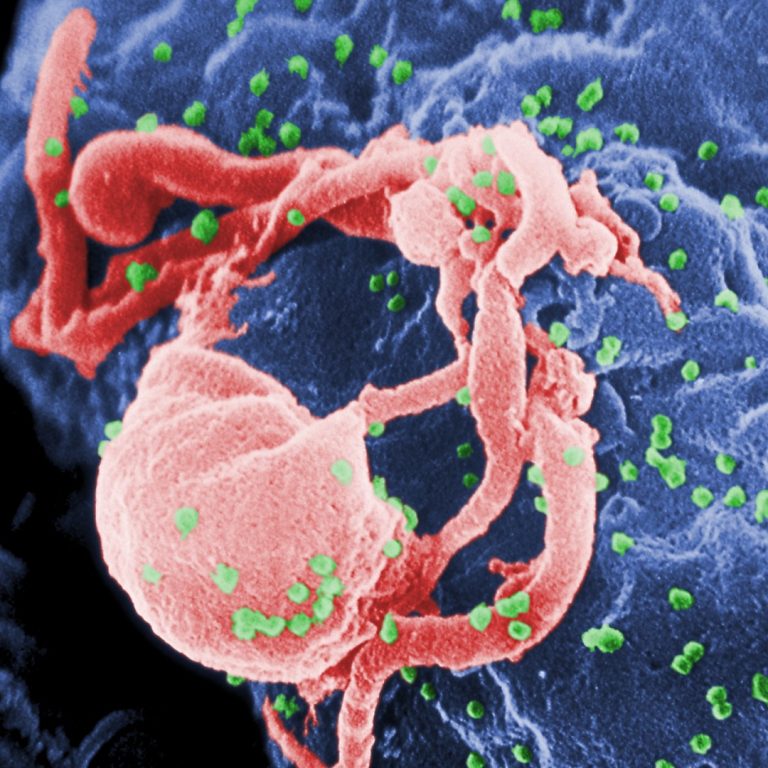
On Jun. 5, 1981, Dr. Michael Gottlieb and colleagues of University of California at Los Angeles reported a…

In 1981, the U.S. Centers for Disease Control and Prevention (CDC) began collecting reports of influenza outbreaks from…
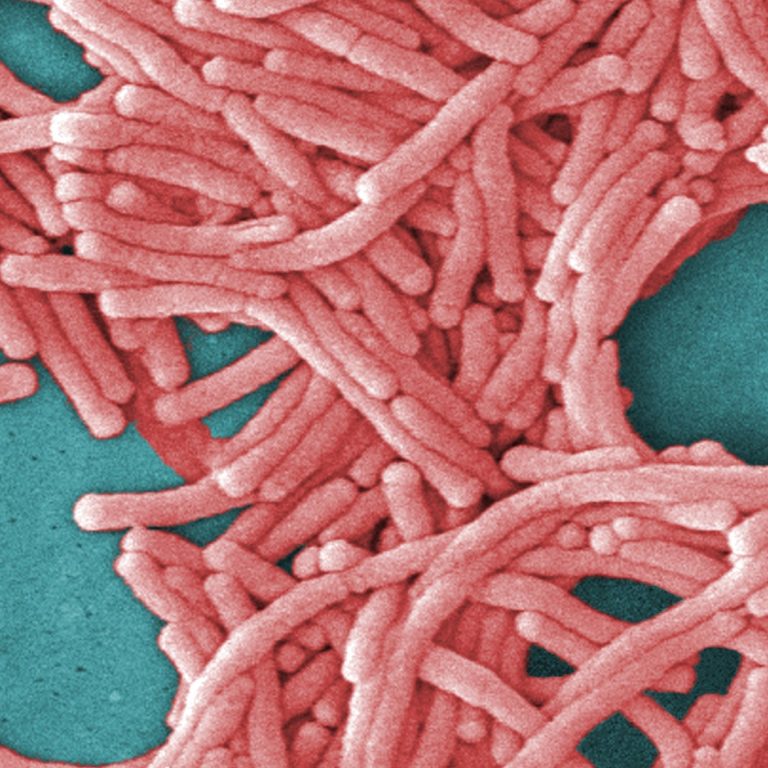
On Nov. 13, 1978, the U.S. Centers for Disease Control and Prevention (CDC) held its first international conference…

On Nov. 21, 1977, the U.S. Food and Drug Administration (FDA) licensed the first pneumococcal vaccine containing 14…
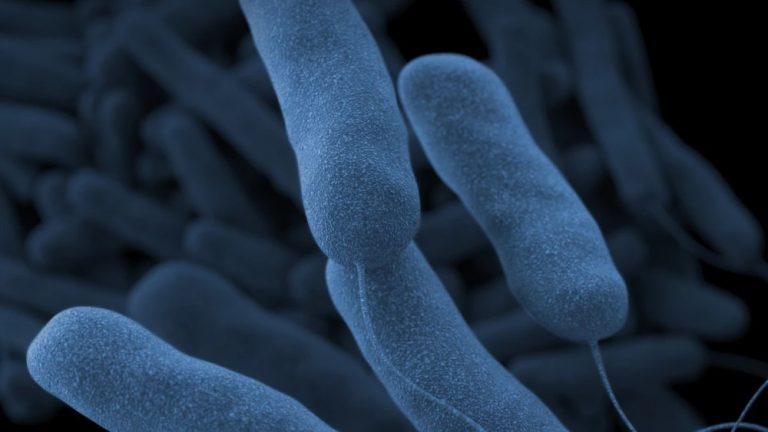
On Jan. 18, 1977, microbiologist Dr. Joseph McDade at the U.S. Centers for Disease Control and Prevention (CDC)…

On Jul. 21, 1976, an estimated 4,400 American Legionnaires and guests gathered at a Bellevue-Stratford Hotel for the…

In 1976, Influenza A/Victoria-like strains had been identified in New Jersey as early as January 21. The novel…