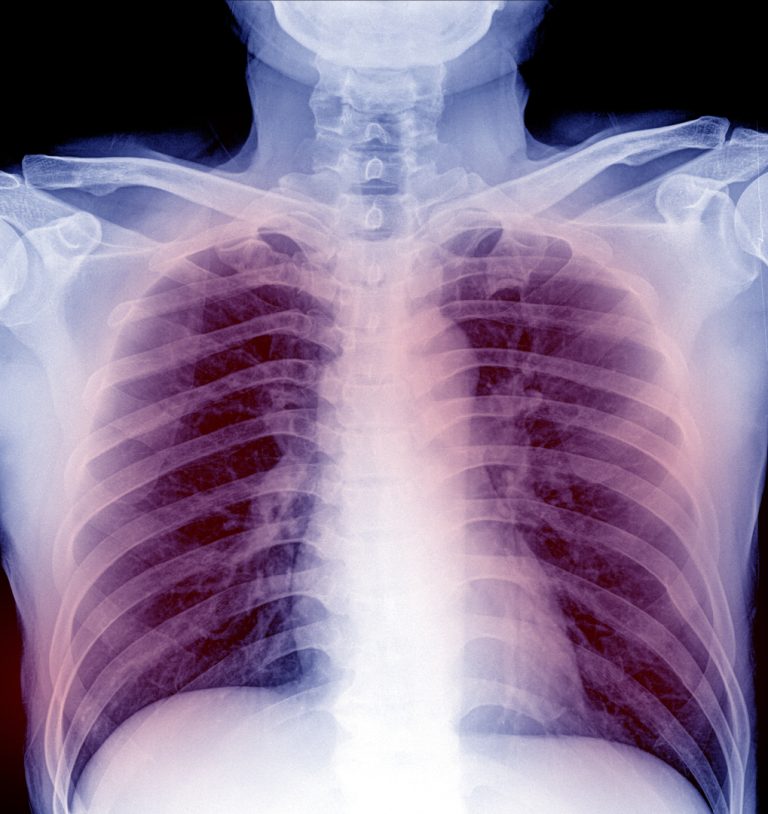
Welder’s Anthrax Treated with Obiltoxaximab in Louisiana, fatalities Reported
On Jan. 1, 2026, the Centers for Disease Control and Prevention (CDC) reported that in September 2024, the…

On Jan. 1, 2026, the Centers for Disease Control and Prevention (CDC) reported that in September 2024, the…
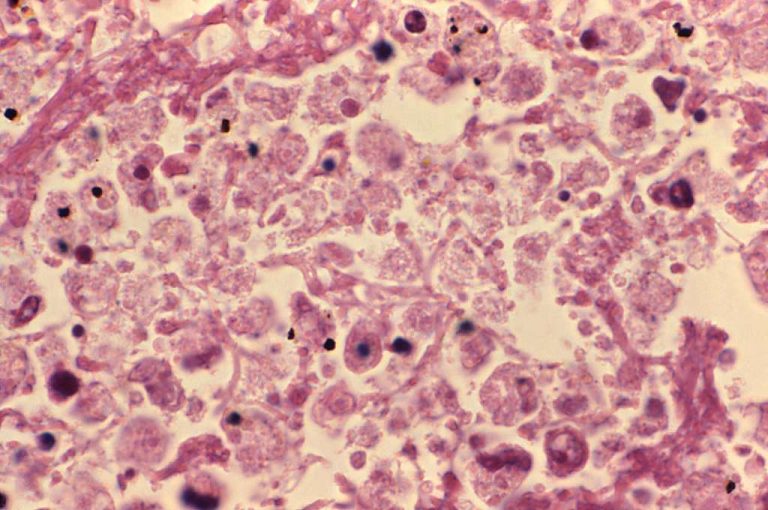
Dec. 4, 2025, new research from the University of Washington (UW) underscores risks of using publicly available large-language…
On Aug. 4, 2025, the New York City Health Department provided an update on the investigation into a…

On Jul. 25, 2025, the New York City Health Department (Health Department) announced an investigation into a community…
On Nov. 5, 2024, a World Health Organization (WHO) study published in eBioMedicine named 17 pathogens that regularly…

On Oct. 23, 2024, Pfizer announced that the U.S. Centers for Disease Control and Prevention’s (CDC) Advisory Committee…
On Aug. 29, 2024, a small team of scientists has launched an open-source database for some of the…

On Jun. 17, 2024, Merck announced that the U.S. Food and Drug Administration (FDA) had approved CAPVAXIVE™ (Pneumococcal…

On Apr. 3, 2024, the U.S. Food and Drug Administration approved Basilea Pharmaceutica’s Zevtera (ceftobiprole medocaril sodium for…

On Nov. 23, 2023, the World Health Organization (WHO) announced that it was monitoring data from Chinese surveillance…

On Sept. 14, 2023, the World Health Organization (WHO) reported that as of September 11, 2023, a total…
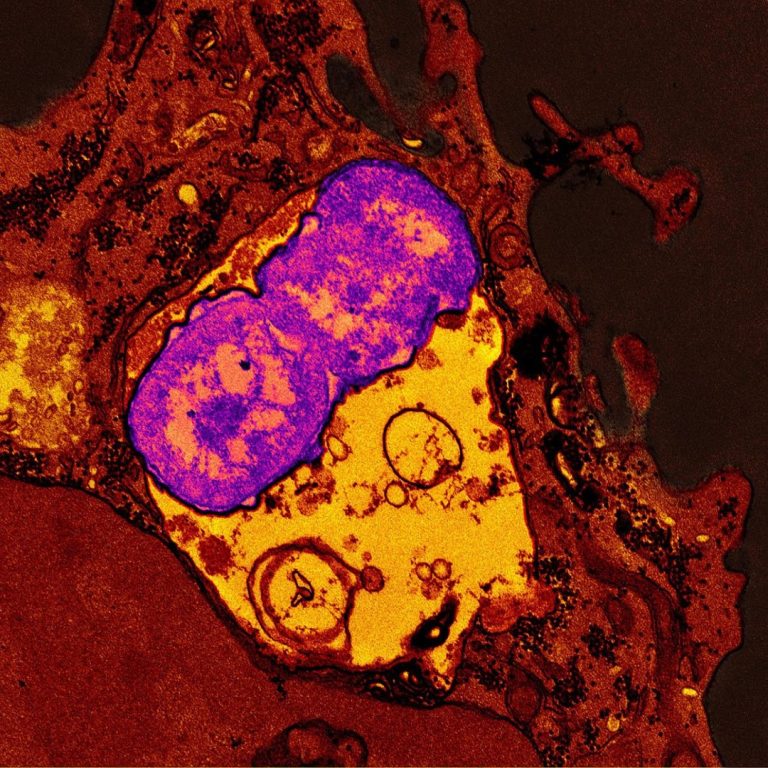
On Sept. 6, 2023, the National Institutes of Health (NIH) announced it was investgating ‘hypervirulent’ strains of the…

On Apr. 14, 2023, the National Health Commission of the People’s Republic of China reported a confirmed case…

On Oct. 24, 2022, Merck announced that the European Commission had approved an expanded indication for VAXNEUVANCEル (Pneumococcal…
On Jul. 22, 2022, Gilead Sciences announced that the Committee for Medicinal Products for Human Use (CHMP) of…

On Jun. 22, 2022, Merck announced that that the U.S. Food and Drug Administration (FDA) had approved an…

On April 21, 2022, researchers at the Centers for Disease Control and Prevention’s (CDC) National Institute for Occupational…

On Apr. 14, 2022, Merck announced that V116, the companyメs investigational 21-valent pneumococcal conjugate vaccine, had received Breakthrough…

On Mar. 31, 2022, Sorrento Therapeutics announced that the FDA had given clearance to commence the Phase 3…

On Feb. 15, 2022, Pfizer announced that the European Medicines Agency had approved the companyメs 20-valent pneumococcal conjugate…

On Dec. 9, 2021, CytoDyn announced that it had submitted a Phase 3, randomized, double blind, placebo controlled…

On Oct. 18, 2021, a NIAID sponsored clinical trial found that treatment with the immunomodulator interferon beta-1a plus…

On Oct. 15, 2021, Merck announced that the European Medicines Agencyメs Committee for Medicinal Products for Human Use…

On Aug. 3, 2021, Vanda Pharmaceuticals announced that enrollment had closed in the ODYSSEY study comparing tradipitant and…

On Jul. 16, 2021, Merck announced the U.S. Food and Drug Administration (FDA) had approved VAXNEUVANCE (Pneumococcal 15-valent…

On Jun. 21, 2021, Cumberland Pharma released a series of case reports describing the effectiveness of Vibativ (telavancin)…

On Jun. 16, 2021, Pfizer and The Academic Research Organization (ARO) from the Hospital Israelita Albert Einstein announced…

On Jun. 8, 2021, Pfizer announced that the U.S. Food and Drug Administration (FDA) had approved PREVNAR 20ル…

On Apr. 21, 2021,Trevena announced that TRV027, the Companyメs novel AT1 receptor selective agonist, had been selected for…

On Feb. 26, 2021, Pfizer announced that the European Medicines Agency (EMA) accepted for review the Marketing Authorization…