University Hospital Basel, Switzerland transfused first two COVID-19 patients with INTERCEPT treated Coronavirus convalescent plasma
On Apr. 2, 2020, Cerus announced that the University Hospital Basel transfused the first two COVID-19 patients with…

On Apr. 2, 2020, Cerus announced that the University Hospital Basel transfused the first two COVID-19 patients with…

On Apr. 2, 2020, Cerus announced that the International Society of Blood Transfusion (ISBT) Working Party on Global…

On Apr. 2, 2020, Emergent BioSolutions announced it had entered into a formal partnership with the U.S. government…
On Apr. 2, 2020, BioAegis Therapeutics announced that it was preparing to evaluate plasma gelsolin replacement in severe…

On Mar. 26, 2020, Cerus announced it had formed a collaborative research group with the aim of optimizing…

On Mar. 23, 2020, researchers at Washington University School of Medicine in St. Louis announced they were investigating…
On Mar. 23, 2020, CSL announced that it was exploring development of a hyperimmune serum that could be…

On Mar. 17, 2020, a small study of macaques found they don’t develop a coronavirus infection the second…
On Dec. 12, 2019, BioAegis Therapeutics announced publication of new research findings with recombinant human plasma gelsolin in…

On Dec. 4. 2019, US-based Nanomix announced that the company had received CE Mark for its S1 Assay…
On Sept. 15, 2017, the U.S. Centers for Disease Control and Prevention (CDC) published updated GamaSTAN S/D prescribing…

On Jun. 17, 2014, Grifols opened state-of-the-art North Fractionation Facility (NFF) in Clayton, NC, where production capacity of…

On May 20, 2010, researchers at the J. Craig Venter Institute in Maryland published results describing the successful…

On Feb. 28, 2002, the FDA announced it had licensed the first nucleic acid test (NAT) system intended…

In February 1986, the U.S. Food and Drug Administration (FDA) approved Hybritech’s Tandem-R PSA assay (P850048) with calibration…
On Feb. 8, 1984, Elmer R. Pfefferkorn published his discovery that treatment of human fibroblasts with human recombinant…

On Jun. 22, 1983, the American Association of Blood Banks, the Council of Community Blood Centers, and the…

On Jan. 4, 1983, epidemiologic evidence that the AIDS agent was blood-borne led to official meetings and public…
In 1982, the first hepatitis B viral vaccines, developed by Merck and also by the Pasteur Institute, were…

In 1981, the U.S. Food and Drug Administration (FDA) approved a plasma-derived hepatitis B vaccine for human use….

On Apr. 1, 1976, physician and scientist C. Ronald Kahn from Harvard University announced he had discovered alterations…

In 1972, Cook County Hospital became the first hospital to use an all frozen blood banking system. This…

In 1972, the Food and Drug Administration’s (FDA) new Bureau of Biologics began to regulate all 7000 U.S….

In 1966, pioneering work in inductively coupled plasma spectroscopy at Iowa State University led to an analysis tool…
In 1964, plasmapheresis was introduced as a means of collecting plasma for fractionation.

In 1953, William P. Murphy, Jr., an American doctor working with colleague Carl Walter, developed the blood bag…

In 1947, Velmer A. Fassel, an American chemist who developed the inductively coupled plasma, received in Ph.D. from…

In 1944, the use of dried plasma became vital to treating wounded solders in World War II.

In 1941, Velmer A. Fassel, an American chemist who developed the inductively coupled plasma, received a B.A. degree…
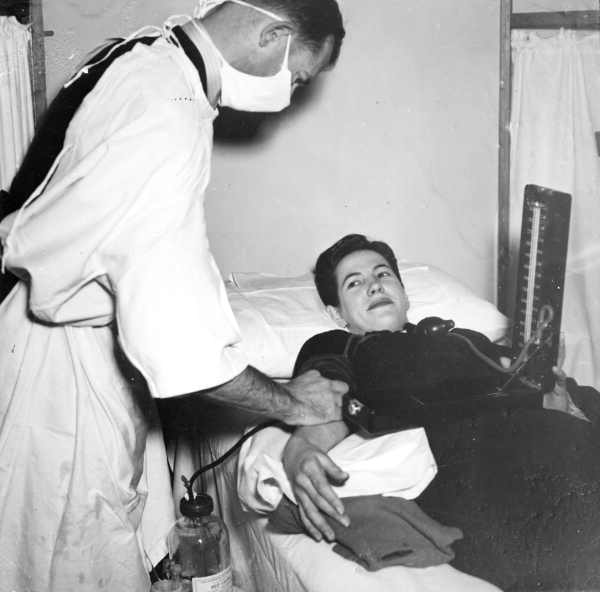
In 1940, the U.S. government established a national blood collection program. That same year the National Research Council…