Roche to invest $50 billion in pharmaceuticals and diagnostics in the U.S. over the next five years
On Apr. 22, 2025, Roche announced that it will invest USD $50 billion into the United States in…

On Apr. 22, 2025, Roche announced that it will invest USD $50 billion into the United States in…
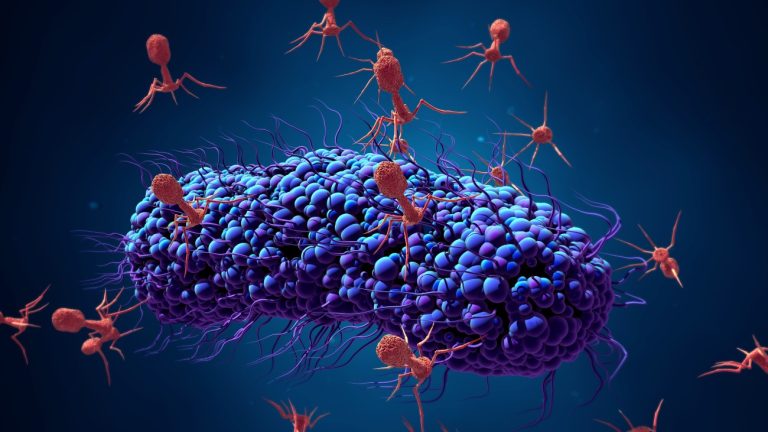
On Apr. 17, 2025, researchers from the University of of Pittsburgh announced they have produced the most detailed…

On Dec. 26, 2024, the Oregon Department of Agriculture (ODA) announced it is alerting pet owners that samples…

On Oct. 31, 2024, researchers from the University of Pittsburgh reported results of a study that showed drug-related…

On Aug. 23, 2024, the U.S. Centers for Disease Control and Prevention (CDC) reported that two human infections…

On Aug. 6, 2024, the University of Pennsylvania announced it had sued German biotechnology firm BioNTech n Pennsylvania…
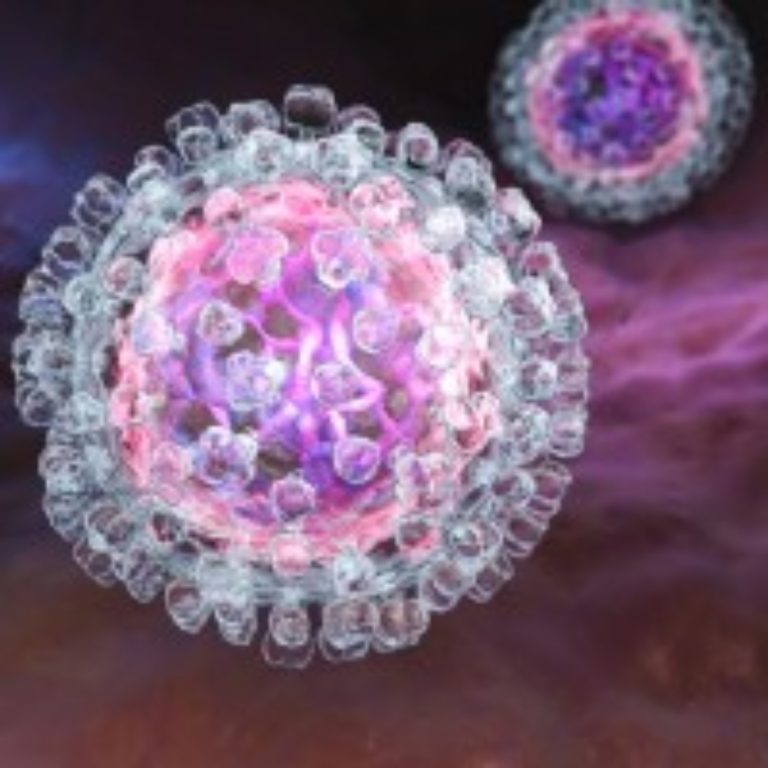
On Jul. 10, 2024, The World Health Organization (WHO) announced it had has prequalified the first hepatitis C…

On Mar. 29, 2024, the U.S. Centers for Disease Control and Prevention (CDC) reported the first U.S. human…
On Jul. 28, 2023, COVID vaccine uptake among hospital workers at a Philadelphia teaching hospital rose from 86%…

On Nov. 16, 2022, the Cancer Prevention and Research Institute of Texas (CPRIT) approved $12 million in recruitment…

On Oct. 27, 2022, Inovio Pharma announced that it has discontinued its internally funded efforts to develop INO-4800…
On May 17, 2022, OraSure Technologies announced that its InteliSwabᆴ COVID-19 rapid tests detect the Omicron BA.2, BA.2.12.1,…

On Apr. 16, 2022, the U.S. Department of Agriculture’s (USDA) Animal and Plant Health Inspection Service (APHIS) confirmed…

On Feb. 2, 2022, a new analysis of the first two patients treated in a clinical trial with…
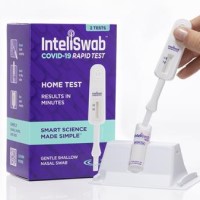
On Jan. 13, 2022, OraSure Technologies that its InteliSwabᆴ COVID-19 rapid tests detected the Omicron variant as effectively…
On Nov. 30, 2021, Inovio Pharma announced the company was rapidly moving to evaluate its COVID-19 DNA vaccine…

On Nov. 9, 2021, Inovio Pharma announced that the U.S. Food and Drug Administration (FDA) provided authorization to…

On Nov. 3, 2021, Inovio Pharma announced that it had received authorization from India’s Central Drugs Standard Control…

On Nov. 2, 2021, OraSure Technologies announced that the EUA for its InteliSwabル COVID-19 rapid tests had been…

On Oct. 30, 2021, Penn Medicine officially opened the doors of its 1.5 million-square-foot future-ready Pavilion as clinical…

On Oct. 21, 2021, Inovio Pharma announced the signing of a non-binding memorandum of understanding with Colombia’s Ministry…

On Oct. 12, 2021, Inovio Pharmaceuticals announced online preprint publication in MedRxiv of Phase 1 clinical data on…

On Oct. 12, 2021, National Resilience announced a strategic collaboration with Children’s Hospital of Philadelphia (CHOP) to implement…

On Oct. 11, 2021, Inovio Pharmaceuticals announced that it had received authorization from Colombia’s INVIMA, to conduct the…

On Sept. 29, 2021, the University of Pennsylvania announced that the Breakthrough Prize in Life Sciences was awarded…
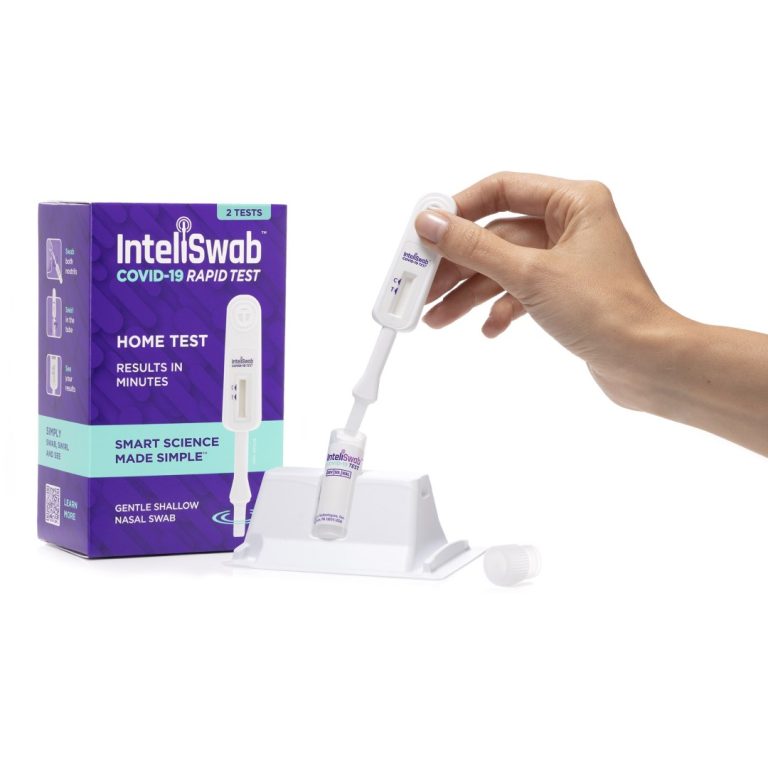
On Sept. 23, 2021, OraSure Technologies and the Biomedical Advanced Research Development Authority (BARDA) announced up to $13.6…

On Sept. 22, 2021, Inovio Pharmaceuticals announced that it had received authorization from COFEPRIS, the national health regulatory…

On Sept. 17, 2021, the U.S. Department of Defense in coordination with the Department of Health and Human…

On Aug. 26, 2021, Inovio Pharma announced that it had received regulatory authorization from Brazil’s ANVISA (Agencia Nacional…

On Aug. 9, 2021, Inovio Pharma announced that it had received regulatory allowance for two clinical trials investigating…