Exact Sciences Launches Cancerguard™, First-of-Its-Kind Multi-Cancer Early Detection Blood Test
On Sept. 10, 2025, Exact Sciences announced the launch of the Cancerguard™ test, a new multi-cancer early detection…

On Sept. 10, 2025, Exact Sciences announced the launch of the Cancerguard™ test, a new multi-cancer early detection…

On Jan. 2, 2025, an international study led by researchers at Karolinska Institutet in Sweden shows that AI-based…

On Oct. 4, 2024, researchers from Oxford university announced they had been awarded funding from Cancer Research UK…

On Sept. 18, 2024, researchers from the Wellcome Sanger Institute and their collaborators reported that they had identified…

On Mar. 18, 2024, the U.S. Environmental Protection Agency (EPA) announced a final rule to prohibit ongoing uses…

On Aug. 31, 2023, the U.S. Food and Drug Administration (FDA) cleared for marketing the updated 23andMe Personal…

On Nov. 16, 2022, the Cancer Prevention and Research Institute of Texas (CPRIT) approved $12 million in recruitment…

On Jun. 21, 2022, Anixa Biosciences announced the publication of a peer-reviewed journal article in Clinical and Experimental…

On Nov. 4, 2020, McGill University Professor William Foulkes awarded 2020 Wilder-Penfield Prize for research in the genetics…

On Apr. 6, 2020, the National Cancer Institute awarded of $14.54 million to Roswell Park Comprehensive Cancer Center…

On Jan. 22, 2019, GlaxoSmithKline acquired TESARO based in Waltham, Massachusetts, for an aggregate cash consideration of approximately…

On Jan. 18, 2019, Masonic Cancer Center researchers received $1.4 million in grants for ovarian cancer research from…

On Mar. 6, 2018, the U.S. Food and Drug Administration authorized 23andMe’s Personal Genome Service Genetic Health Risk…

On May 8, 2013, was the first World Ovarian Cancer Day. On this day, ovarian cancer organizations from…

On Oct. 16, 2006, the Cancer Genome Atlas program, created by National Cancer Institute and the National Human…

On Jul. 17, 2006, Eli Lilly and Company’s Gemzar was approved by the U.S. Food and Drug Administration (FDA) for…

On Jun. 5, 2003, data from the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial gave fresh insight…

On Jul. 17, 2002, a National Cancer Institute funded trial showed that postmenopausal women who used estrogen replacement…

On Feb. 7, 2002, scientists from the National Cancer Institute (NCI) and the Food and Drug Administration (FDA)…

On Jul. 1, 1996, topotecan (Hycamptin), the first of a class of drugs that interferes with the enzyme…

On Oct. 7, 1994, a strong candidate for the 17q-linked BRCA1 gene, which influences susceptibility to breast and…

In 1993, the Prostate, Lung, Colorectal, and Ovarian trial was launched. The trial was designed to determine whether…

In 1993, the Washington University School of Medicine announced that it was a participating site for the prostate,…

On Dec. 18, 1992, Taxol (paclitaxel), an anticancer drug extracted from the bark of the Pacific yew, received…

In 1992, the National Cancer Institute (NCI) awarded the Pacific Health Research Institute its largest and most enduring…

On Mar. 3, 1989, carboplatin (Paraplatin), a drug related to cisplatin, was approved by the U.S. Food and…

On Mar. 3, 1989, the U.S. Food and Drug Administration (FDA) approved Bristol-Myers’ PARAPLATIN (carboplatin) for the treatment…
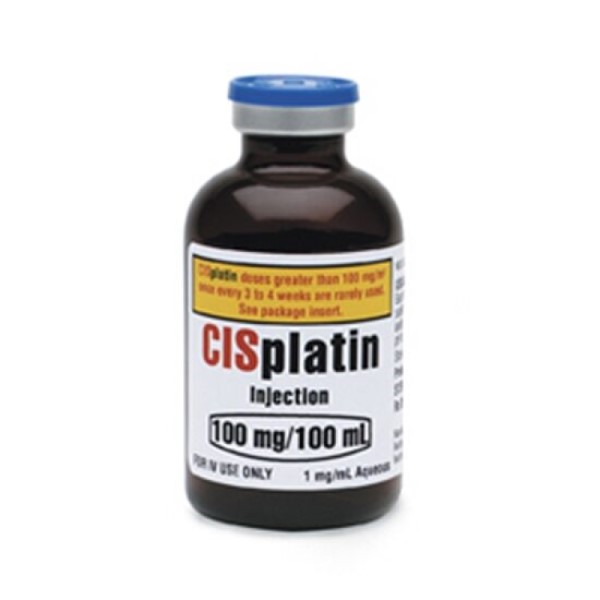
On Dec. 19, 1978, the U.S. Food and Drug Administration (FDA) approved cisplatin (Platinol) for use in combination…

In 1970, Cisplatin, a platinum-containing anticancer compound with unique biologic effects, entered clinical trials. On Dec. 19, 1978,…

In 1967, E.R. Squibb & Sons delved into cancer research, discovering and developing hydroxyurea for leukemia and advanced…