Roche to invest $50 billion in pharmaceuticals and diagnostics in the U.S. over the next five years
On Apr. 22, 2025, Roche announced that it will invest USD $50 billion into the United States in…

On Apr. 22, 2025, Roche announced that it will invest USD $50 billion into the United States in…

On Apr. 10, 2025, Novartis, a leading global innovative medicines company, announced a planned $23 billion investment over…

On Mar. 25, 2025, Merck announced it has signed a licensing agreement for a heart disease drug with…
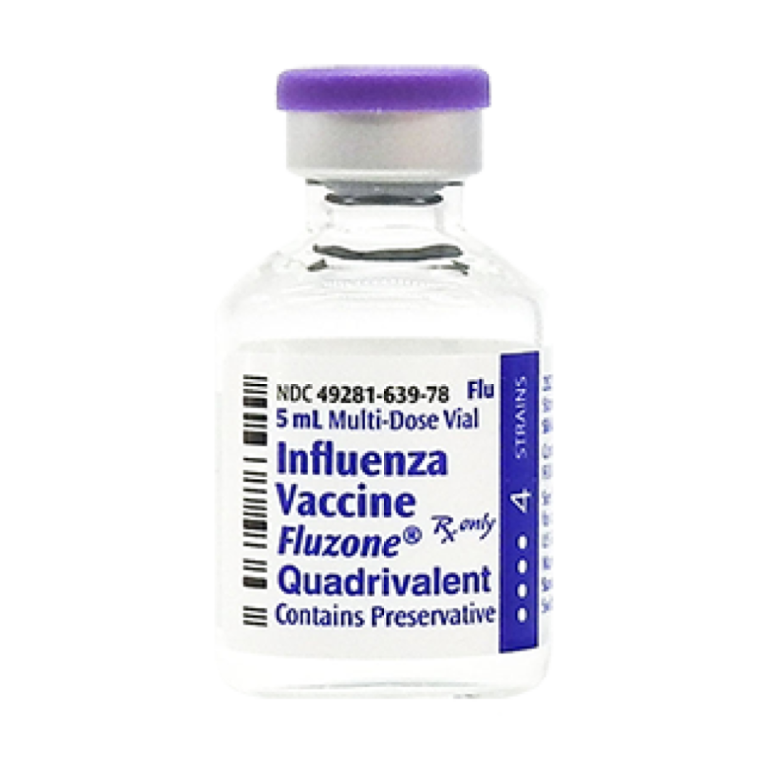
On Mar. 14, 2025, Sanofi announced the immediate adoption of influenza strains selected by the U.S. Food and…
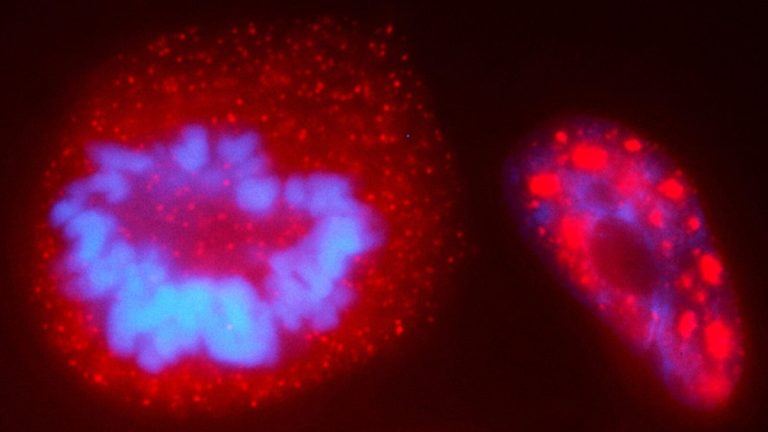
On Sep. 9, 2024, a Princeton University team announced they had developed a way to probe chromosomes and…

On Dec. 10, 2022, CSL announced data affirming the long-term durability and safety of single-infusion HEMGENIX® (etranacogene dezaparvovec-drlb)…

On May 18, 2022, the U.S. Department of Agriculture’s (USDA) Animal and Plant Health Inspection Service (APHIS) confirmed…

On Apr. 27, 2022, the U.S. Department of Agricultureメs Animal and Plant Health Inspection Service announced it had…

On Jun. 1, 2021, Sanofi announced that Vaxelis (Diphtheria and Tetanus Toxoids and Acellular Pertussis, Inactivated Poliovirus, Haemophilus…

On Mar. 10, 2021, the U.S. Department of Defense (DOD) announced that it had identified additional personnel authorized…

On Jul. 30, 2020, Seqirus announced it had begun shipping its portfolio of seasonal influenza vaccines to customers…

On May 8, 2020, the FDA issued an emergency use authorization (EUA) to Rutgers Clinical Genomics Laboratory for…

On Apr. 21, 2020, Rutgers University announced it has launched the nation’s largest prospective study of health care…

On Apr. 21, 2020,the fight against the coronavirus is underway in a Rutgers UniversityヨCamden research laboratory, where chemistry…

On Apr. 13, 2020, Pluristem Therapeutics announced it has treated its first patient suffering from COVID-19 complications in…

On Mar. 25, 2020, Merck announced it had donated 300,000 masks to New Jersey’s Office of Homeland Security…

On Mar. 24, 2020, T2 Biosystems announced it has entered into a worldwide licensing agreement for a rapid…

On Mar. 24, 2020, T2 Biosystems announced it had entered into a worldwide licensing agreement for a rapid…

On Mar. 23, 2020, Acura Pharmaceuticals announced that Abuse Deterrent Pharma, its licensee of LTX-03 (Hydrocodone Bitartrate and…
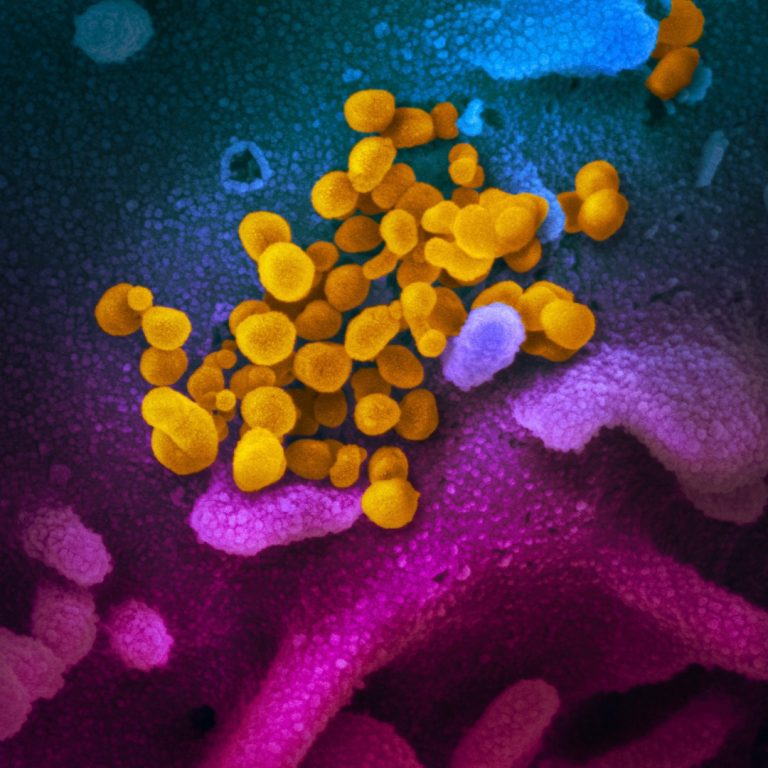
On Mar. 17, 2020, the virus that causes coronavirus disease 2019 (COVID-19) was stable for several hours to…

On Mar. 20, 2020, BioReference Labs, an OPKO Health company, announced a collaboration with the State of New…

On Feb. 24, 2020, Seqirus announced that the U.S. Food and Drug Administration (FDA) had approved the first…
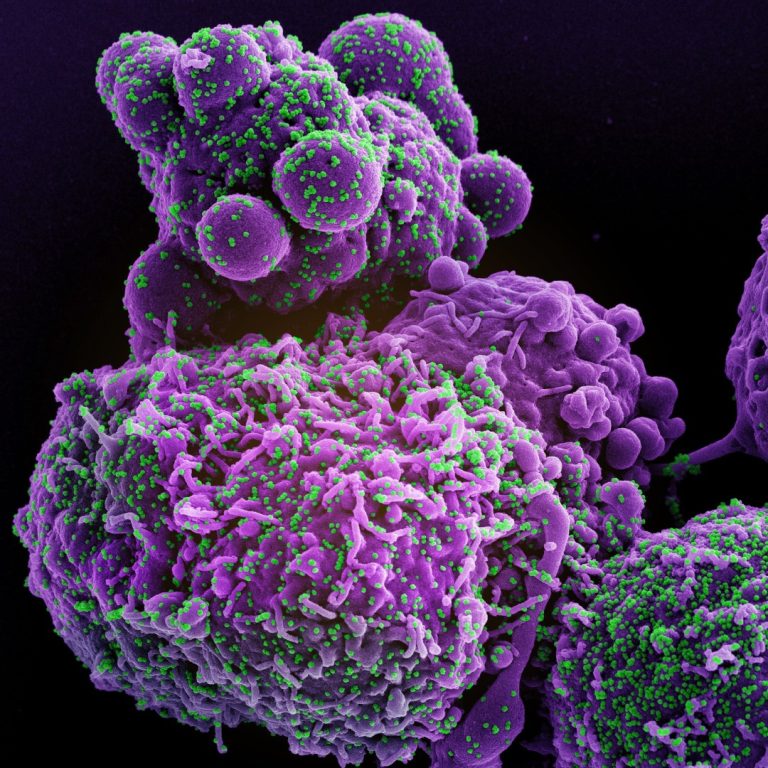
On Jan. 29, 2020, Sorrento Therapeutics announced it had initiated a clinical and manufacturing collaboration with Celularity, a…

On Dec. 11, 2019, Seqirus announced that its cell-based quadrivalent influenza vaccine (QIVc) had received approval from Health…

On Dec. 5, 2018, Seqirus, a CSL subsidiary, announced real-world data showing that its cell-based quadrivalent influenza vaccine…

On Jul. 6, 2018, Teva Pharmaceuticals announced it would move its U.S. headquarters to Parsippany-Troy Hills, New Jersey…

In 2018, the Lombardi Comprehensive Cancer Center became the 16th National Cancer Institute-designated Comprehensive Cancer Center funded with…

On Oct. 21, 2015, the Omar Boraie Chair in Genomic Science was established at Rutgers Cancer Institute. The…

On Sept. 18, 2015, Stephen J. Elledge from Brigham and Women’s Hospital and Evelyn M. Witkin from Rutgers…

On May 5, 2015, Rutgers Cancer Institute of New Jersey was one of seven international sites to offer…