“Milestones in Fluoride” Cartoon Illustrating the Facts behind Natural Mineral Published
On Mar. 24, 2025, LifeScienceHistory.com published the cartoon “Milestones in Fluoride” illustrating the facts behind the naturally occurring…

On Mar. 24, 2025, LifeScienceHistory.com published the cartoon “Milestones in Fluoride” illustrating the facts behind the naturally occurring…

On Mar. 14, 2025, the Michigan Department of Health and Human Services (MDHHS) and Oakland County Health Division…
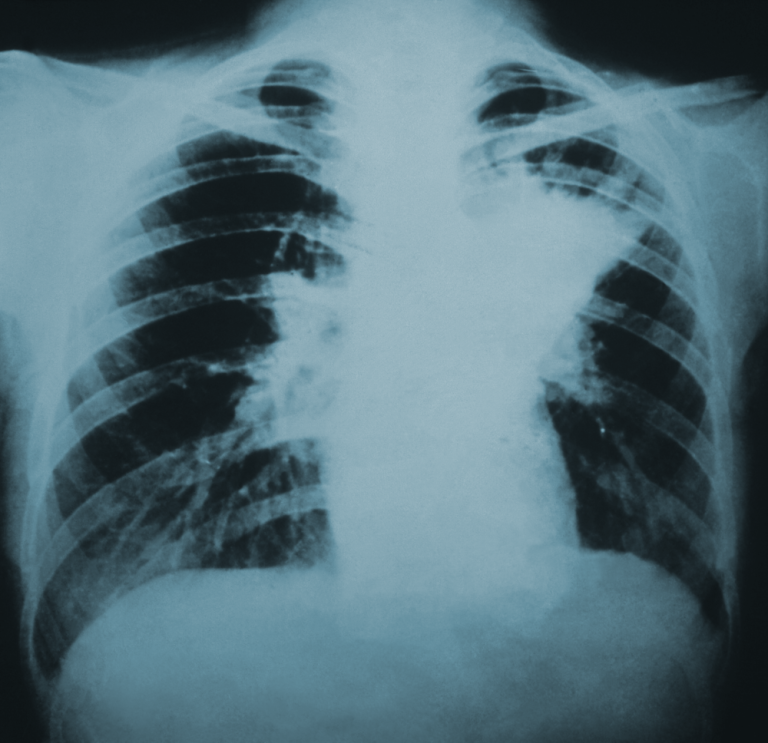
On Dec. 31, 2024, the U.S. Centers for Disease Control and Prevention released a study of the largest…

On Dec. 26, 2024, the Oregon Department of Agriculture (ODA) announced it is alerting pet owners that samples…

On Dec. 18, 2024, the Barbara Ann Karmanos Cancer Institute announced that the new TheraBionic P1 device, an…

On Aug. 26, 2024, the University of Michigan (UM) announced that one in five parents say their child…
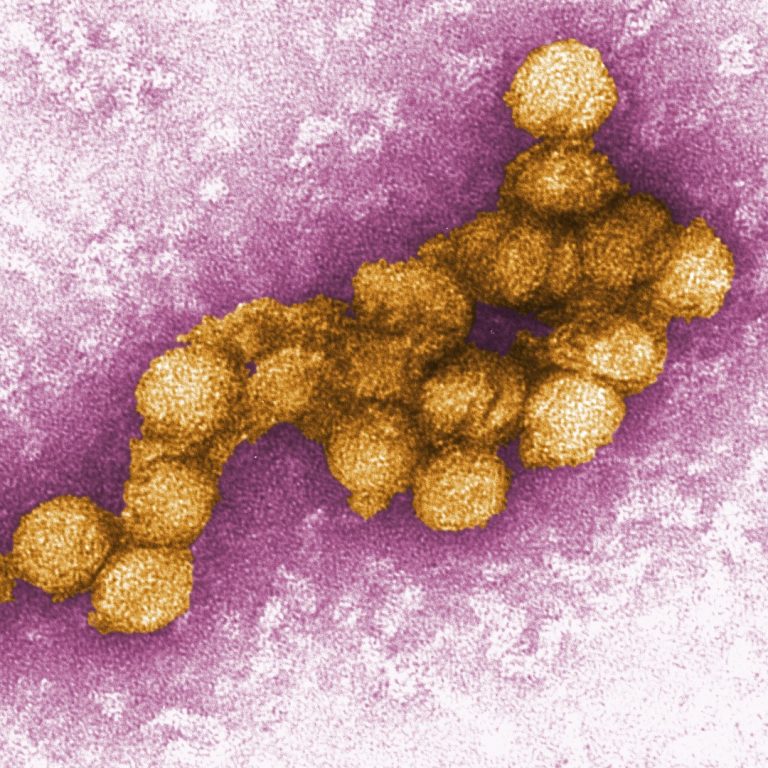
On Aug. 15, 2024, the Oakland County Health Division in Pontiac, Michigan urged residents to protect themselves from mosquito…

On Jul. 29, 2024, a team led by the Barbara Ann Karmanos Cancer Institute and the Wayne State…

On Jul. 9, 2024, the Michigan Department of Agriculture and Rural Development (MDARD) announced the detection of highly…

On May 20, 2024, the Michigan Department of Agriculture and Rural Development (MDARD) announced the detection of highly…

On Apr. 2, 2024, the U.S. Department of Agriculture (USDA) confirmed the detection of highly pathogenic avian influenza…
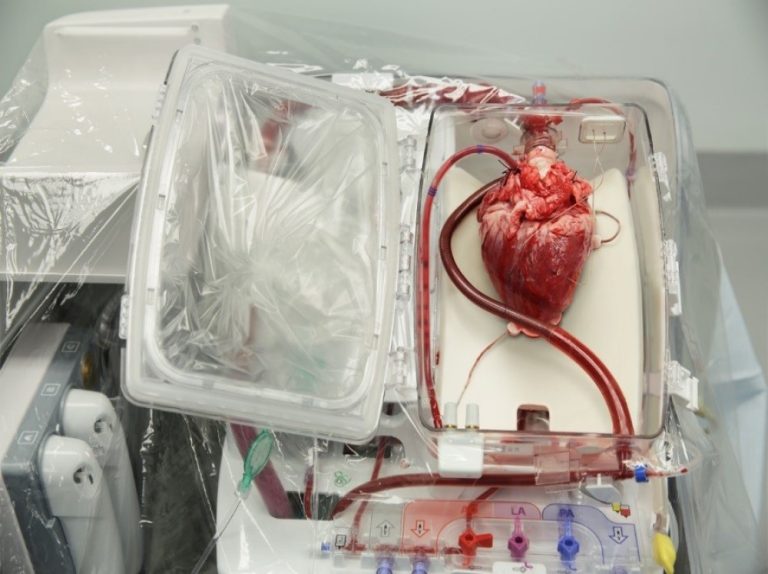
On Aug. 22, 2023, a study led by researchers at Washington University School of Medicine in St. Louis…

On Aug. 4, 2023, the U.S. Centers for Disease Control and Prevention (CDC) reported the first two U.S….

On Mar. 16, 2023, Pfizer announced that the U.S. Food and Drug Administration’s Antimicrobial Drugs Advisory Committee (AMDAC)…
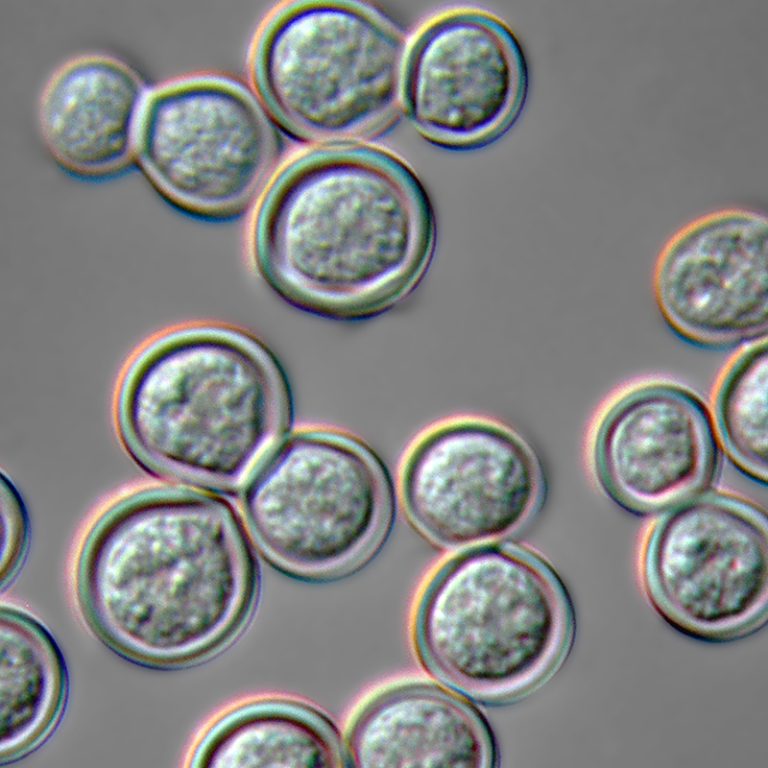
On Feb. 28, 2023, Public Health Delta & Menominee Counties (PHDM) was alerted to three cases of atypical…

On Oct. 26, 2022, the Barbara Ann Karmanos Cancer Institute announced it had completed an over 50,000-square-foot expansion…

On Supt. 22, 2022, Pfizer announced an agreement to supply up to six million treatment courses of its…

On Aug. 29, 2022, the U.S. Department of Health and Human Services (HHS) announced it had provided approximately…
On Jun. 30 2022, Pfizer announced the submission of a New Drug Application (NDA) to the U.S. Food…

On Jun. 14 2022, Pfizer reported data from the Phase 2/3 EPIC-SR (Evaluation of Protease Inhibition for COVID-19…

On Jun. 6, 2022, Pfizer further strengthened its commitment to United States manufacturing with a $120 million investment…

On Mar. 22, 2022, Pfizer announced an agreement with UNICEF to supply up to 4 million treatment courses…

On Mar. 9, 2022, Pfizer announced that it had initiated a Phase 2/3 study, EPIC-PEDS (Evaluation of ProteaseInhibition…

On Jan. 24, 2022, Anixa Biosciences announced that the company and its partner, MolGenie, had synthesized a compound…

On Jan. 4, 2022, Pfizer announced that the U.S. government had committed to purchasing an additional 10 million…

On Dec. 22, 2021, Pfizer announced that the U.S. Food and Drug Administration (FDA) had authorized the emergency…

On Dec. 14, 2021, Pfizer announced final results from an analysis of all 2,246 adults enrolled in its…

On Nov. 18, 2021, Pfizer announced an agreement with the U.S. government to supply 10 million treatment courses…

On Nov. 16, 2021, Pfizer announced it had submitted an Emergency Use Authorization (EUA) of its investigational oral…

On Nov. 5, 2021, Pfizer announced it was investigational novel COVID-19 oral antiviral candidate, PAXLOVID, significantly reduced hospitalization…