U.S. completes withdrawal from World Health Organization
On Jan. 22, 2026, The U.S. has finalized its withdrawal from the World Health Organization (WHO), one year…

On Jan. 22, 2026, The U.S. has finalized its withdrawal from the World Health Organization (WHO), one year…

On Dec. 4, 2025, the World Health Organization (WHO) released the 2025 World Malaria Report showing that since…

On Dec. 2, 2025, findings in the latest Global Burden of Disease 2023 study published in The Lancet…

On Nov. 25, 2025, recent studies published in PNAS have revealed that many mosquito species regularly engage in…

On Oct. 7, 2025, the World Health Organization (WHO) launched the Global Clinical Trials Forum (GCTF), a global,…
On Sept. 24, 2025, Indian drugmakers Dr Reddy’s Laboratories and Hetero Labs announced they will sell generic versions…

On Sept. 22, 2025, The Gates Foundation will give $912 million to the Global Fund to Fight AIDS,…
On Aug. 6, 2025, the Tacoma-Pierce County Health Department (TPCHD) reported an East Pierce County, Washington woman who…
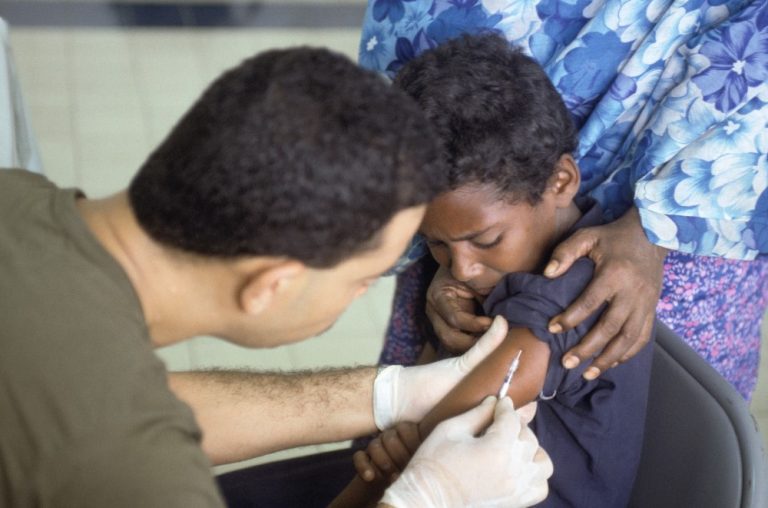
On Jul. 15, 2025, in 2024, 89% of infants globally – about 115 million – received at least…

On Jul. 14, 2025, researchers at Burnet Institute, in collaboration with Gavi, the Vaccine Alliance, have provided the…

On Jul. 9, 2025, a research team led by Eske Willerslev, professor at the University of Copenhagen and…

On Jul. 8, 2025, Novartis announces it had received approval in Switzerland for Coartem Baby, which it said…

On Jun. 18, 2025, researchers at the University of Washington (UW) School of Medicine announced that a highly…

On May 29, 2025, The World Health Organization (WHO), Africa Centres for Disease Control and Prevention (Africa CDC)…

On May 8, 2025, Bill Gates announced that he has decided to give away his money back to…

On May 1, 2025, the U.S. National Institutes of Health (NIH), the world’s largest funder of biomedical research,…

On Apr. 22, 2025, Syngenta announced that its next-generation insecticide Sovrenta® has received pre-qualification by World Health Organization…
On Apr. 17, 2025, a study was published that shows the United States spent roughly US$12 billion on…

On Feb. 5, 2025, the proposed dismantling of the U.S. Agency for International Development (USAID) could disrupt clinical…

On Jan. 23, 2025, the World Health Organization (WHO) announced that following a nearly century-long effort, Georgia has…

On Dec. 11, 2024, the World Health Organization (WHO) reported new data has revealed that an estimated 2.2…

On Nov. 5, 2024, a World Health Organization (WHO) study published in eBioMedicine named 17 pathogens that regularly…
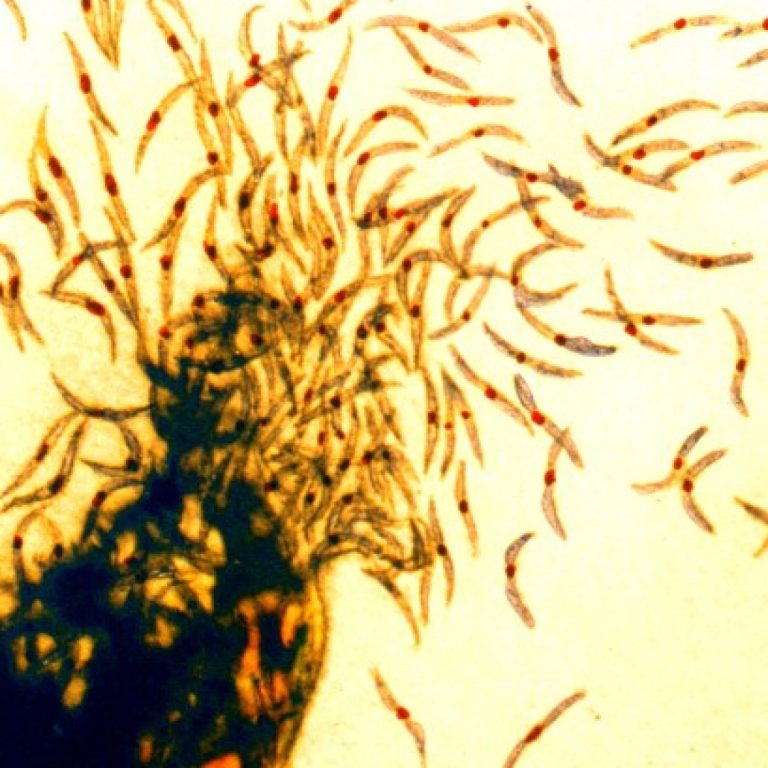
On Oct. 20, 2024, the World Health Organization (WHO) announced it had certified Egypt as malaria-free, marking a…
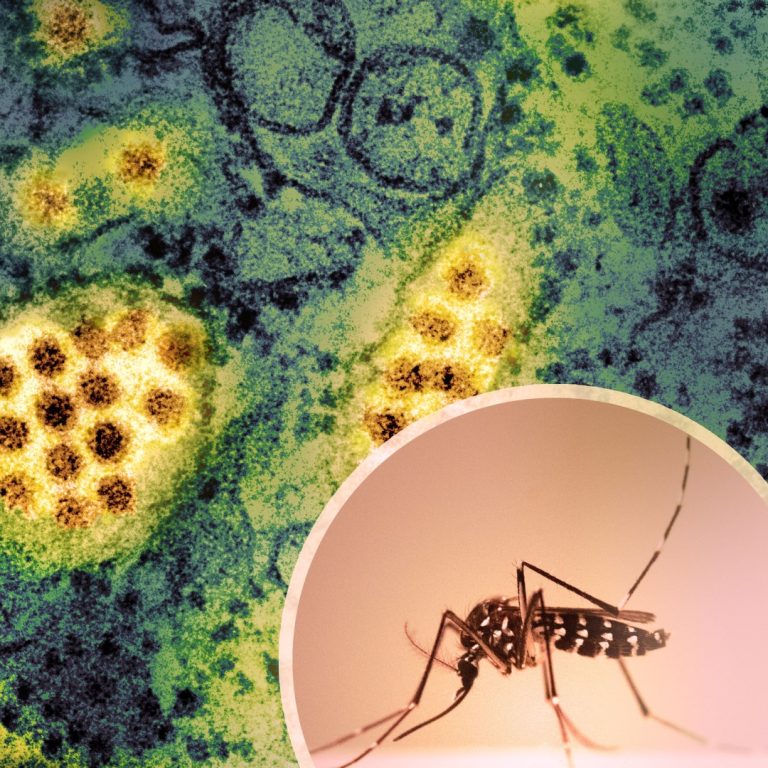
On Sept. 19, 2024, Oxitec announced it had launched Sparks™, a new commercial platform to accelerate the global…

On Jan. 12, 2024, the World Health Organization (WHO) announced it had certified Cabo Verde as a malaria-free…

On Dec. 21, 2023, the World Health Organization (WHO) announced that it had prequalified the R21/Matrix-M malaria vaccine…
On Oct. 2, 2023, the World Health Organization (WHO) announced it had recommended a new vaccine, R21/Matrix-M, developed…
On Aug. 24, 2023, NIAID reported that emerging resistance to common malaria treatments in Uganda could be connected…

On Aug. 18, 2023, the Maryland Department of Health has confirmed and reported a positive case of locally…

On Jun. 26, 2023, the Centers for Disease Control and Prevention (CDC) announced they were collaborating with U.S….