The FDA approved Gleevec for Five Rare, Life-Threatening Disorders
On Oct. 19, 2006, the U.S. Food and Drug Administration (FDA) approved the drug, Gleevec developed by Oregon…

On Oct. 19, 2006, the U.S. Food and Drug Administration (FDA) approved the drug, Gleevec developed by Oregon…
In 2006, Calistoga Pharmaceuticals was founded as a Seattle-based spin-off from ICOS Corp. that was dedicated to developing…

On May 14, 2002, President George W. Bush signed the Hematologic Cancer Research Investment and Education Act of…

On Feb. 1, 2002, the U.S. Food and Drug Administration (FDA) approved the drug Gleevec, formerly known as…

On Nov. 7, 2001, the Oregon Health Sciences University Foundation (OHSU) announced that the Klamath Falls-based JELD-WEN Foundation…
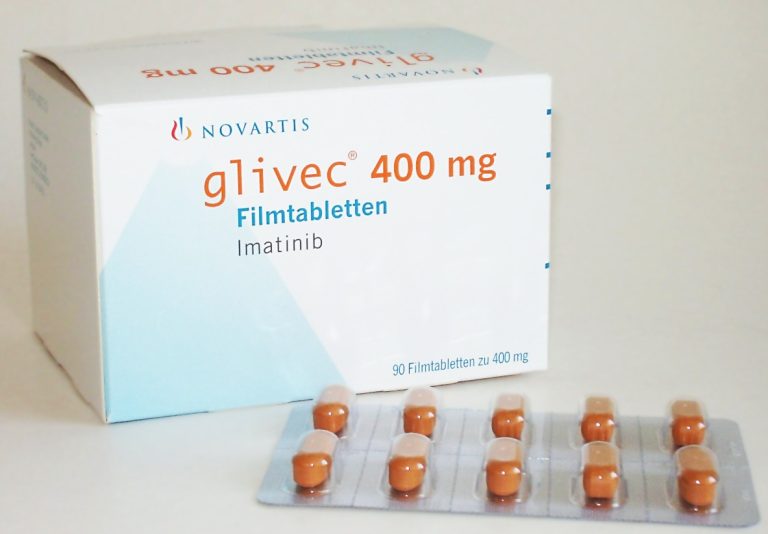
On May 10, 2001, the U.S. Food and Drug Administration (FDA) approved Gleevec, the world’s first targeted cancer…
In 2001, The J.P. McCarthy Cord Stem Cell Bank at the Karmanos Cancer Institute was founded as a…

In 2001, the drug imatinib mesylate (Gleevec) was shown in a clinical trial to be effective against chronic…

In 2000, researchers from Lawrence Berkeley, Lawrence Livermore, and Los Alamos national laboratories at the Joint Genome Institute…
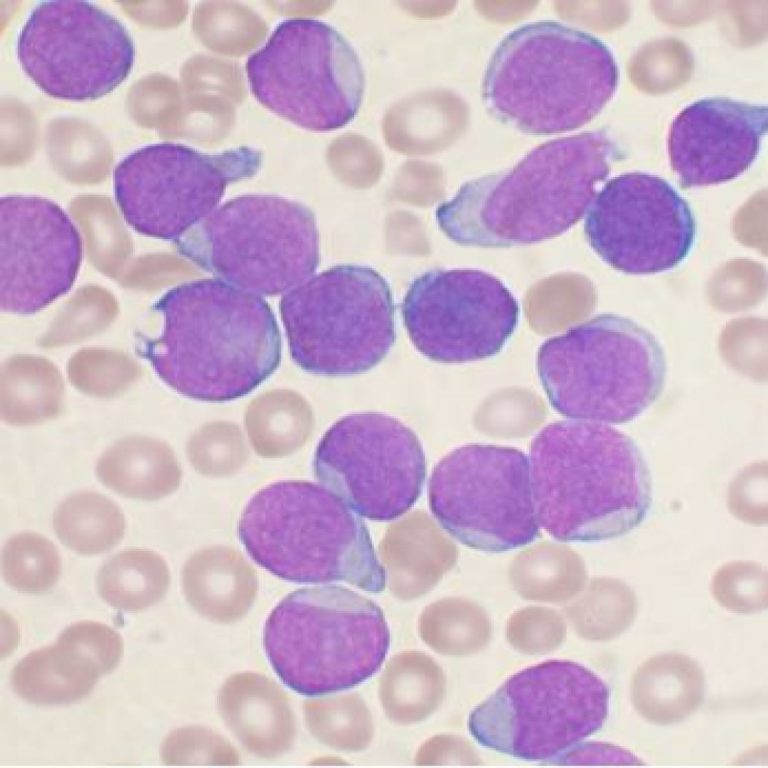
In 1997, cancer stem cells were first identified in acute myelogenous leukemia. They will later be identified in…

In 1997, the National Cancer Institute (NCI) and Chinese scientists found that occupational exposure to benzene is associated…

In 1997, a mouse model of promyelocytic leukemia was developed by Washington University – St. Louis researcher Timothy…

On Dec. 9, 1995, the U.S. Food and Drug Administration (FDA) approved tretinoin, a differentiating agent related to…

In 1994, Washington University – St. Louis reseacher Lee Ratern, MD and colleagues announced they had developed an…

In 1992, the Oregon Cancer Center, later known as the OHSU Cancer Institute, was founded at Oregon Health…

In 1988, Gertrude B. Elion shared the Nobel Prize in Physiology or Medicine for the ‘discoveries of important…

In 1987, The Cold Spring Harbor Laboratory (CSHL) Cancer Center received National Cancer Institute (NCI) designation. CSHL researchers…
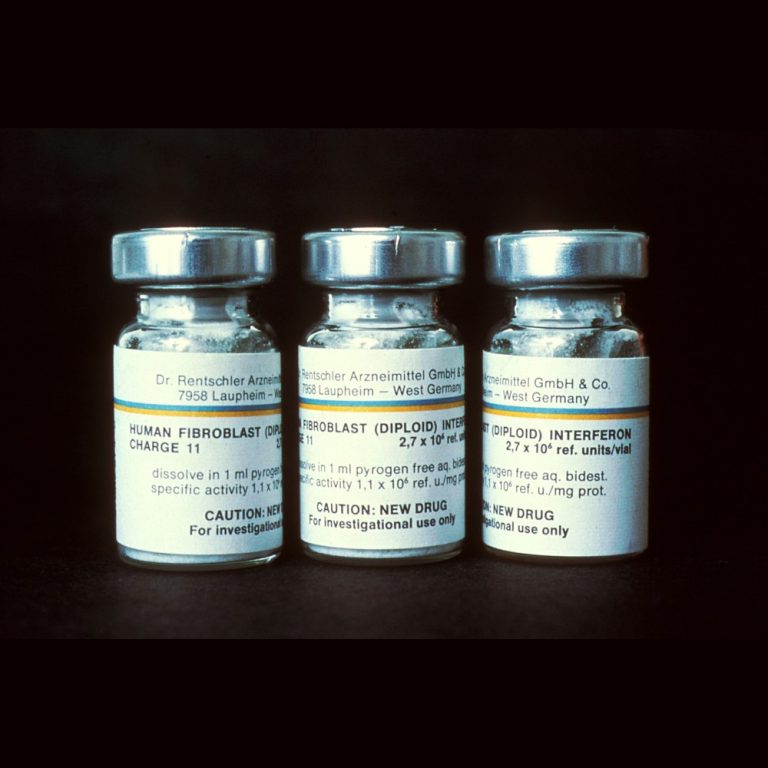
On Jun. 5, 1986, the U.S. Food and Drug Administration (FDA) approved Hoffmann La Roche’s Interferon initially for…

In 1980, National Cancer Institute (NCI) scientists from the Gallo group isolated human T-cell lymphotrophic virus 1 (HTLV-1)….
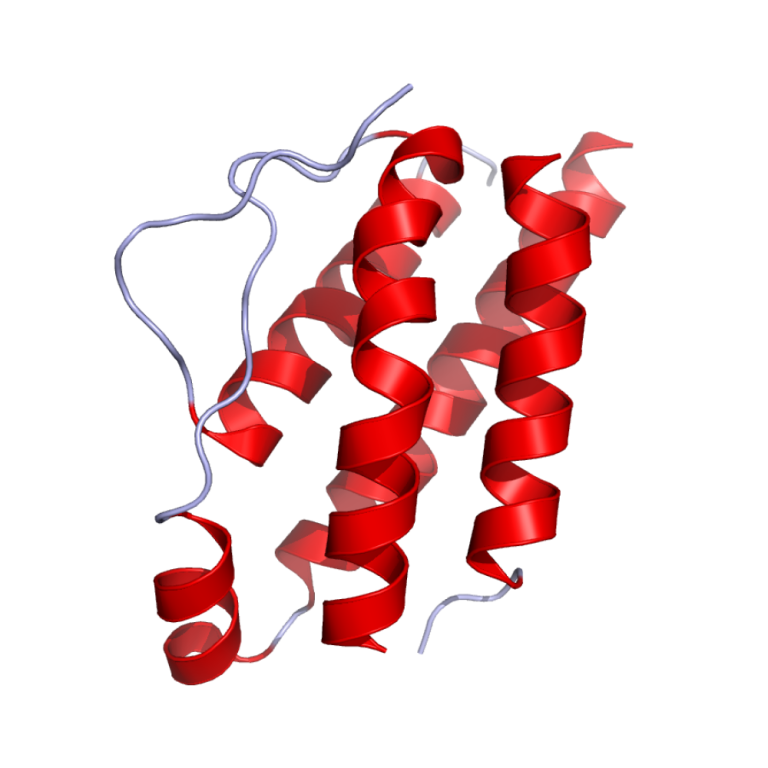
In 1976, interleukin-2 was discovered and purified which allowed researchers to grow T-cells and study their immunology, which…
In 1975, Dr. E. Donnall Thomas, a pioneer in bone marrow transplantation, secured a permanent treatment home at…

In 1968, the world’s first successful bone-marrow transplant was completed at the University of Minnesota Hospital under the…

In 1967, E.R. Squibb & Sons delved into cancer research, discovering and developing hydroxyurea for leukemia and advanced…
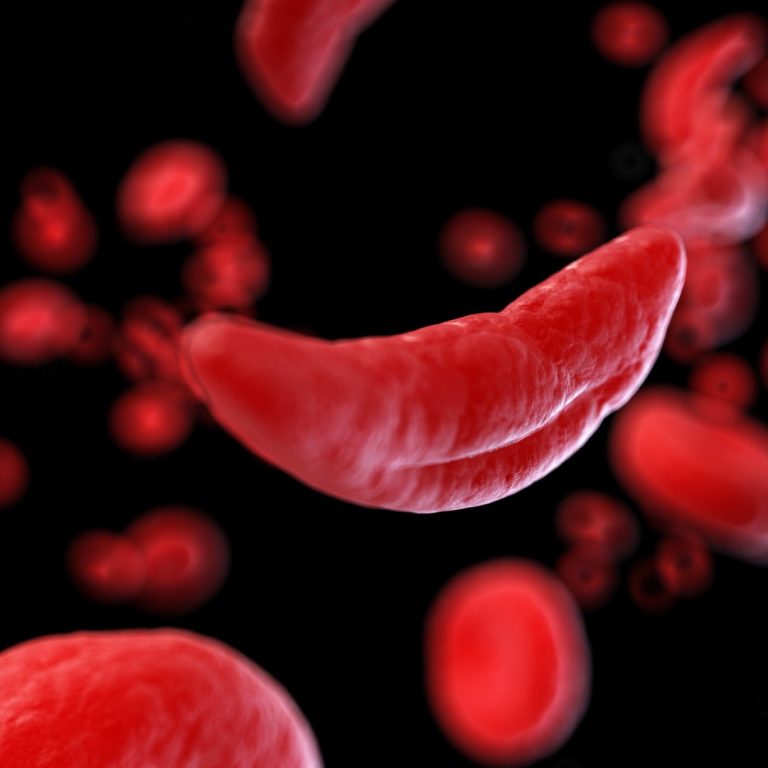
In 1966, a group of St. Jude patients were the first acute lymphoblastic leukemia (ALL) patients to ever…
In 1966, a group of St. Jude patients were the first acute lymphoblastic leukemia (ALL) patients to ever…

On May 7, 1962, the Acute Leukemia Task Force held its first meeting. It focused the combined efforts…

In 1962, the U.S. Centers for Disease Control and Prevention (CDC) performed the first Epidemic Intelligence Service (EIS)…
In 1960, chromosome abnormalities were associated with leukemias.

In 1958, NCI scientists pioneer the use of combination chemotherapy. Partial and complete remissions, as well as prolonged…
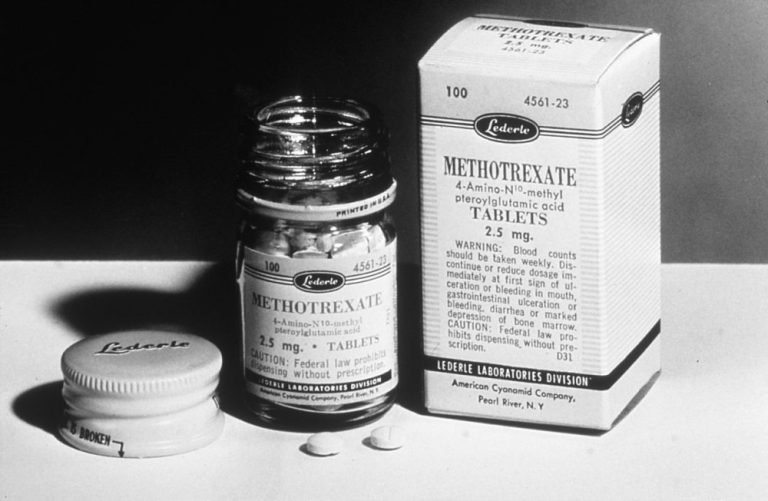
On Dec. 7, 1953, the U.S. Food and Drug Administration (FDA) approved Dava Pharmaceuticals’ methotrexate oral tablet, an…